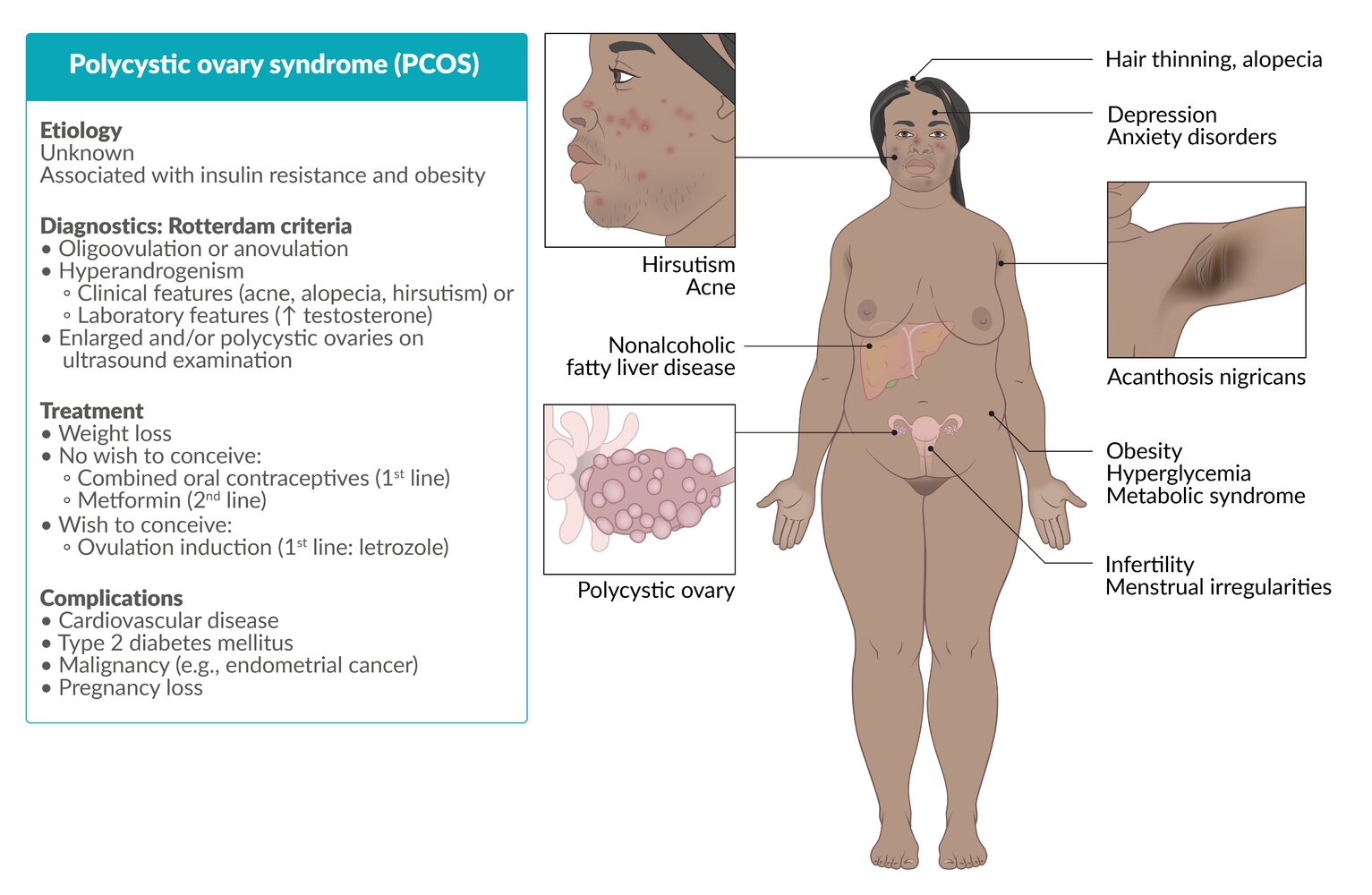
Characterized by hyperandrogenism, oligoovulation/anovulation, and/or the presence of polycystic ovaries
Epidemiology
- Prevalence: 6–12% of women in their reproductive years in the US
Etiology
Pathophysiology
Key points
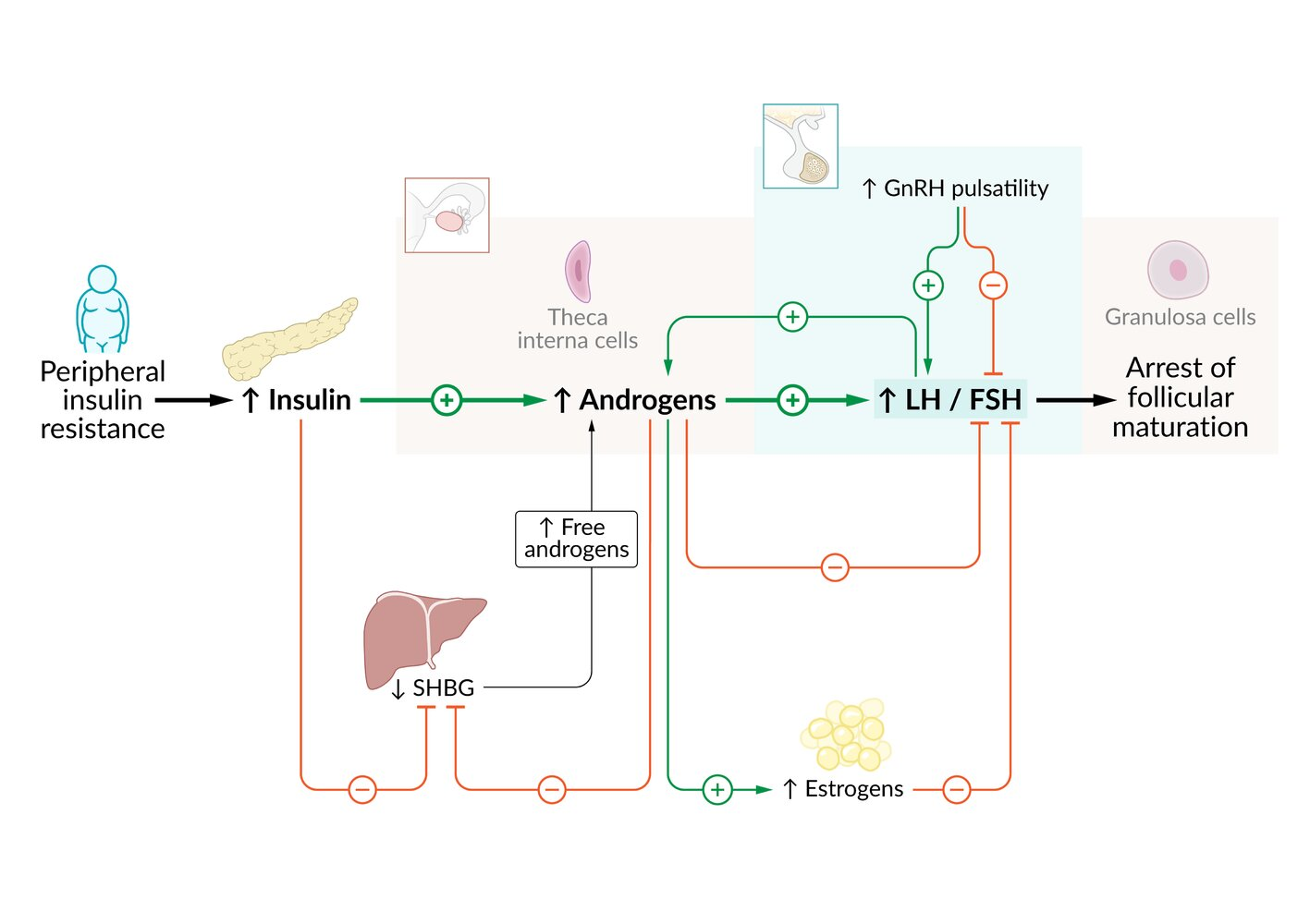
- Core Problem: A self-perpetuating cycle of disordered HPO axis function and insulin resistance.
- Neuroendocrine Defect:
- Abnormal, high-frequency GnRH pulses lead to preferential pituitary secretion of LH over FSH.
- This results in an elevated LH:FSH ratio (>2:1).
- Insulin Resistance & Hyperandrogenism:
- Most patients have insulin resistance (& obesity), causing compensatory hyperinsulinemia.
- High LH + High Insulin synergistically stimulate ovarian theca cells to produce excess androgens (e.g., testosterone).
- Insulin also ↓ liver production of Sex Hormone-Binding Globulin (SHBG), which ↑ free androgen levels.
- Clinical Consequences:
- Hyperandrogenism: Causes hirsutism, acne, and alopecia.
- Anovulation: High intra-ovarian androgens and a relative lack of FSH cause follicular arrest (forming “cysts”) and prevent ovulation, leading to amenorrhea/oligomenorrhea.
- Endometrial Risk: Chronic anovulation leads to unopposed estrogen (no progesterone is made), increasing the risk of endometrial hyperplasia and cancer.
Clinical features
Onset of symptoms typically occurs during adolescence.
- Menstrual irregularities
- Primary or secondary amenorrhea
- Oligomenorrhea
- Menorrhagia
- Infertility or difficulties conceiving
- Insulin resistance and associated conditions
- Metabolic syndrome (especially obesity) → ↑ risk of sleep apnea
- Nonalcoholic fatty liver disease
- Skin conditions
- Hirsutism
- A condition of excessive male pattern hair growth in women (e.g., on the chin, above the upper lip, and around the umbilicus) that is most commonly idiopathic but associated with excess androgen in 10% of cases.
- Androgenic alopecia
- Acne vulgaris
- Oily skin
- Acanthosis nigricans
- Hirsutism
- Psychiatric conditions
- Malignancy (increased risk before menopause): Endometrial cancer
Diagnostics
Treatment
- Target BMI < 25 kg/m2 (can reduce estrone production in the adipose tissue)
Patients not planning to conceive
For patients who do not wish to conceive, the therapeutic goals are to control menstrual irregularities and hyperandrogenism, treat comorbidities, and improve quality of life.
- Combined oral contraceptives (COCs)
- Indication: first-line treatment for hyperandrogenism and/or menstrual cycle abnormalities
- Additional benefits
- ↓ Endometrial hyperplasia → ↓ risk of endometrial carcinoma
- ↓ Menstrual bleeding
- ↓ Acne
- Treatment of hirsutism
- COCs increase the production of SHBG, which binds excess androgen.
- Additional benefits
- Indication: first-line treatment for hyperandrogenism and/or menstrual cycle abnormalities
- Metformin: improves menstrual irregularities, metabolic outcomes, and weight (especially when combined with lifestyle modifications)
Patients planning to conceive
Management of comorbidities (e.g., weight loss for overweight or obese patients) and induction of ovulation.
- Letrozole (off-label): first-line therapy for ovulation induction
- An aromatase inhibitor
- Improves pregnancy and live birth rates in patients with anovulatory infertility with no other causes
- Mechanism of action: aromatase inhibition reduces estrogen production, stimulating FSH secretion and inducing ovulation
- Clomiphene: alternative to letrozole
- Exogenous gonadotropins: The low-dose regimen is the second-line treatment for ovulation induction.
- Agents: exogenous FSH and human menopausal gonadotropin
- Metformin
- Can be used as second-line monotherapy for fertility treatment.
- Combination with clomiphene may increase pregnancy rates, especially in obese women.