Epidemiology
Type 2 DM
- Age
- Adult onset typically > 40 years
- Mean age of onset is decreasing
- Race: highest prevalence in Native Americans, Hispanics, African Americans, and Asian non-Hispanic Americans
Etiology
Type 1 DM
- Autoimmune destruction of pancreatic β cells in genetically susceptible individuals
- HLA association: HLA-DR3 and HLA-DR4 positive patients are at increased risk of developing T1DM.
- Associated with other autoimmune conditions
- Hashimoto thyroiditis
- Type A gastritis
- Celiac disease
- Primary adrenal insufficiency
Classifications
- Type 1: formerly known as insulin-dependent (IDDM) or juvenile-onset diabetes mellitus
- Type 2: formerly known as non-insulin-dependent (NIDDM) or adult-onset diabetes mellitus
- Gestational diabetes
- Other types of diabetes mellitus
- MODY (maturity-onset diabetes of the young): genetic defects leading to β-cell dysfunction
- Different forms of autosomal dominant inherited diabetes mellitus that manifest before the age of 25 years and are not associated with obesity or autoantibodies
- Multiple monogenic subtypes (most common: MODY II due to glucokinase gene defect, and MODY III, due to hepatocyte nuclear factor-1-α gene defect)
- MODY II
- A single mutation leads to impaired insulin secretion due to altered glucokinase function.
- Glucokinase is the glucose sensor of the β cell, facilitating storage of glucose in the liver, especially at high concentrations.
- There is no increased risk of microvascular disease.
- Despite stable hyperglycemia and chronically elevated HbA1C levels, MODY II can be managed with diet alone.
Pathophysiology
Normal insulin physiology
- Secretion: Insulin is synthesized in the β cells of the islets of Langerhans. The cleavage of proinsulin (precursor molecule of insulin) produces C-peptide (connecting peptide) and insulin, which consists of two peptide chains (A and B chains).
- Action
- Carbohydrate metabolism
- Protein metabolism: insulin inhibits proteolysis, stimulates protein synthesis, and stimulates cellular uptake of amino acids
- Lipid metabolism: maintains a fat depot and has an antiketogenic effect
- Electrolyte regulation:
- Stimulates intracellular potassium accumulation
- Directly stimulates Na+/K+ ATPase and promotes intracellular alkalosis, reduces phosphate levels (glucose binds to phosphate in the cell), and stimulates magnesium uptake into cells
- Insulin boosts glucose uptake into cells through two linked processes:
- Direct effect: Insulin stimulates GLUT transporters to move glucose into cells through facilitated diffusion
- Indirect effect: Insulin activates Na⁺/K⁺-ATPase pumps, which:
- Pump Na⁺ out of cells → Creates high Na⁺ outside
- This Na⁺ gradient powers SGLT transporters
- SGLT uses Na⁺ flow into cells to drag glucose in with it (co-transport)
- Reduces phosphate levels: When insulin promotes glucose uptake, glucose must be phosphorylated to glucose-6-phosphate
- Stimulates intracellular potassium accumulation
- Those with long-standing diabetes (ie, >5 years) frequently also have alpha cell failure with decreased glucagon secretion and therefore have an even greater risk of rapid hypoglycemia.
Type 2 diabetes
- Mechanisms
- Peripheral insulin resistance
- Numerous genetic and environmental factors
- Central obesity → increased plasma levels of free fatty acids → impaired insulin-dependent glucose uptake into hepatocytes, myocytes, and adipocytes
- Increased serine kinase activity in fat and skeletal muscle cells → phosphorylation of insulin receptor substrate (IRS)-1 → decreased affinity of IRS-1 for PI3K → decreased expression of GLUT4 channels → decreased cellular glucose uptake
- Numerous genetic and environmental factors
- Pancreatic β cell dysfunction: accumulation of pro-amylin (islet amyloid polypeptide) in the pancreas → decreased endogenous insulin production
- Peripheral insulin resistance
- Progression
- Initially, insulin resistance is compensated by increased insulin and amylin secretion.
- Over the course of the disease, insulin resistance progresses, while insulin secretion capacity declines.
- After a period of impaired glucose tolerance with isolated postprandial hyperglycemia, diabetes manifests with fasting hyperglycemia.
Clinical features
Onset
Type 1 DM
- Often sudden
- Diabetic ketoacidosis (DKA) is the first manifestation in approx. one-third of cases.
Type 2 DM
- Typically gradual
- The majority of patients are asymptomatic.
- Some patients may present with a hyperglycemic crisis.
- Elderly patients especially may present in a hyperosmolar hyperglycemic state.
- Occasionally, patients with T2DM present with DKA , which mostly affects black and Hispanic individuals.
- Symptoms of complications may be the first clinical sign of disease.
Common clinical features
- Classic symptoms of hyperglycemia
- Polyuria, which can lead to secondary enuresis and nocturia in children
- Polydipsia
- Polyphagia
- Nonspecific symptoms
- Unexplained weight loss
- Visual disturbances, e.g., blurred vision
- Fatigue
Specific clinical features
Type 2 DM
- Possible cutaneous signs of insulin resistance
- Benign acanthosis nigricans
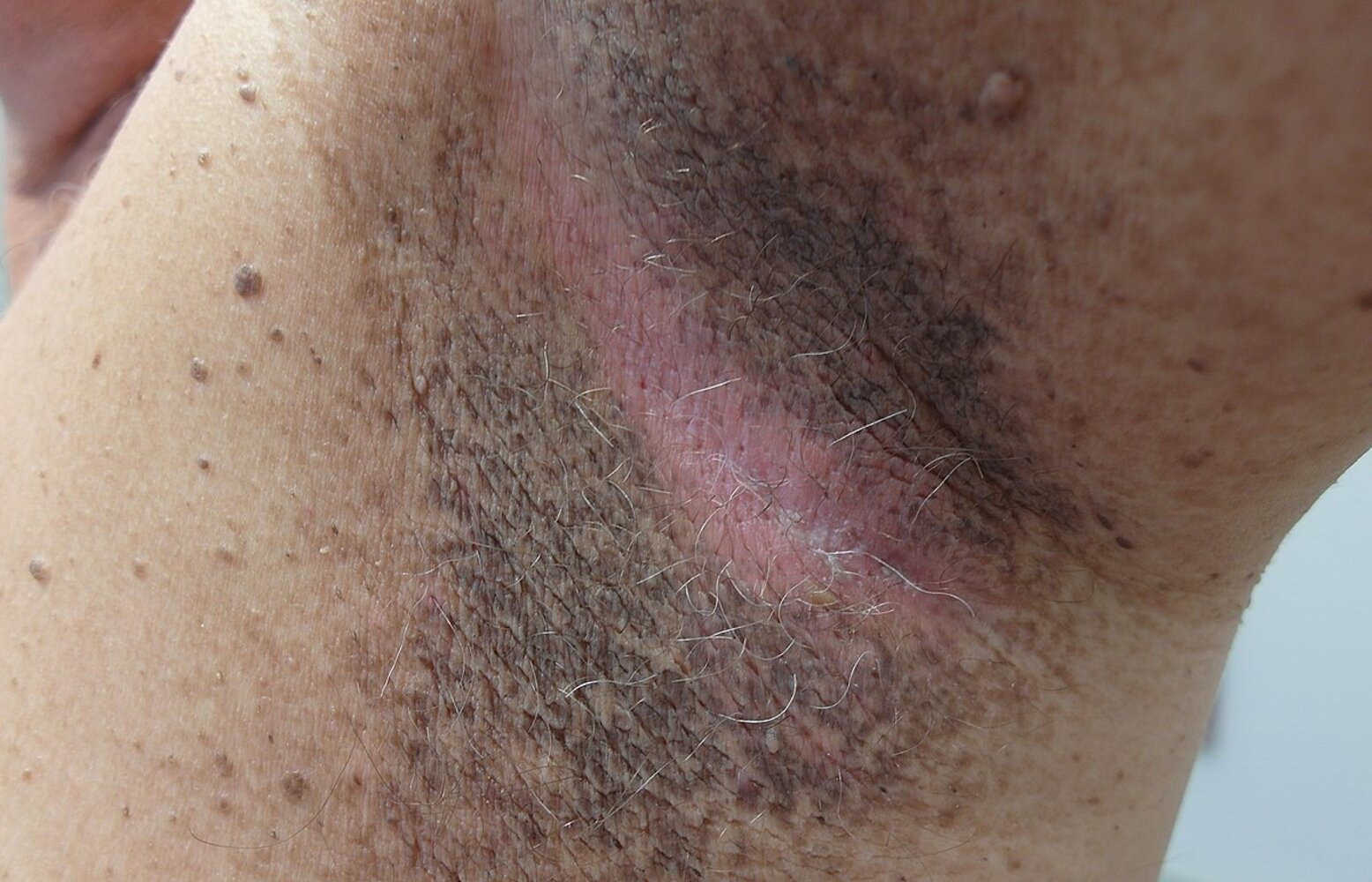
- Acrochordons
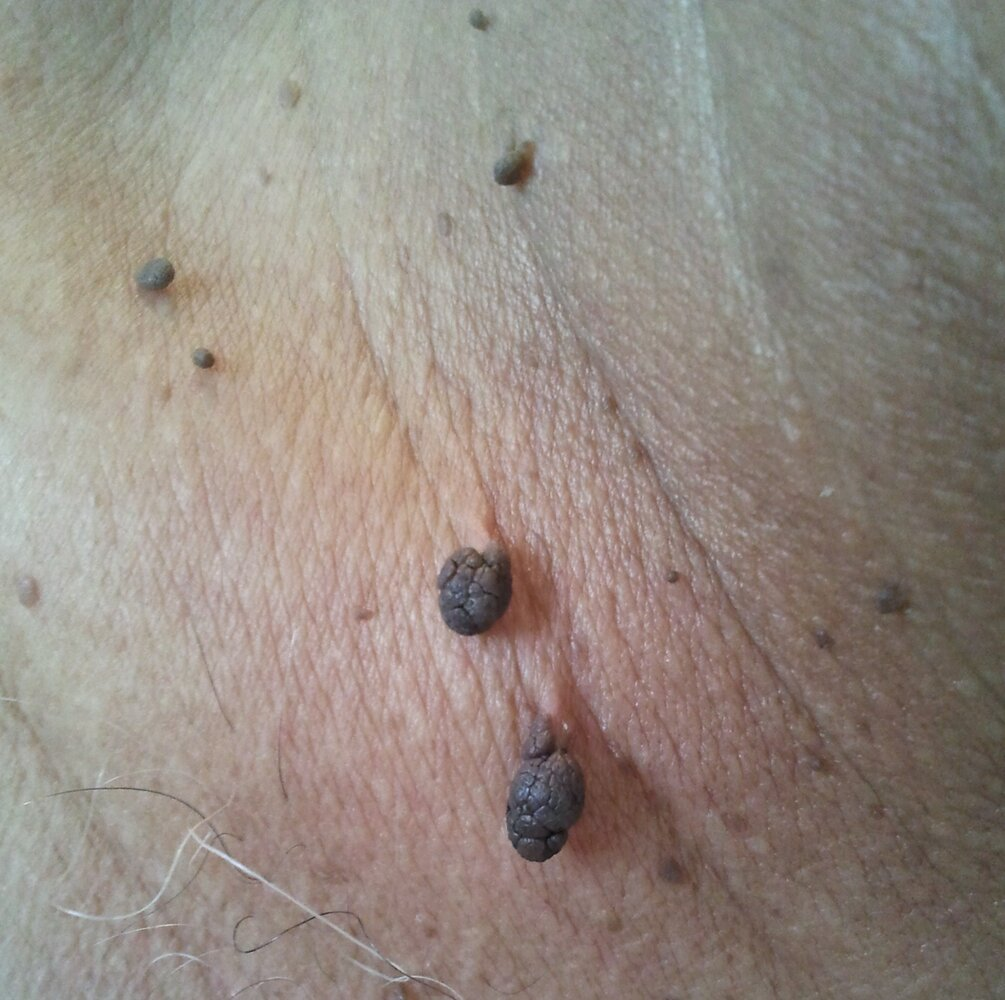
- Benign acanthosis nigricans
Complications
Microvascular disease
- Onset: typically arises 5–10 years after onset of disease
- Pathophysiology: chronic hyperglycemia → nonenzymatic glycation of proteins and lipids → thickening of the basal membrane with progressive function impairment and tissue damage
- Manifestations
- Diabetic nephropathy
- Diabetic retinopathy, glaucoma
- Diabetic neuropathy including diabetic gastroparesis
- Diabetic foot
Tip
Strict glycemic control is crucial in preventing microvascular disease.
Other complications
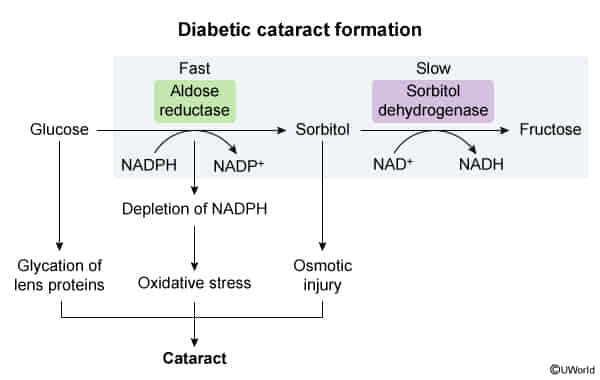
- Osmotic damage: occurs in tissues with high aldolase reductase activity and low/absent sorbitol dehydrogenase activity (e.g., eyes, peripheral nerves) → diabetic cataracts, neuropathy
Diagnostics
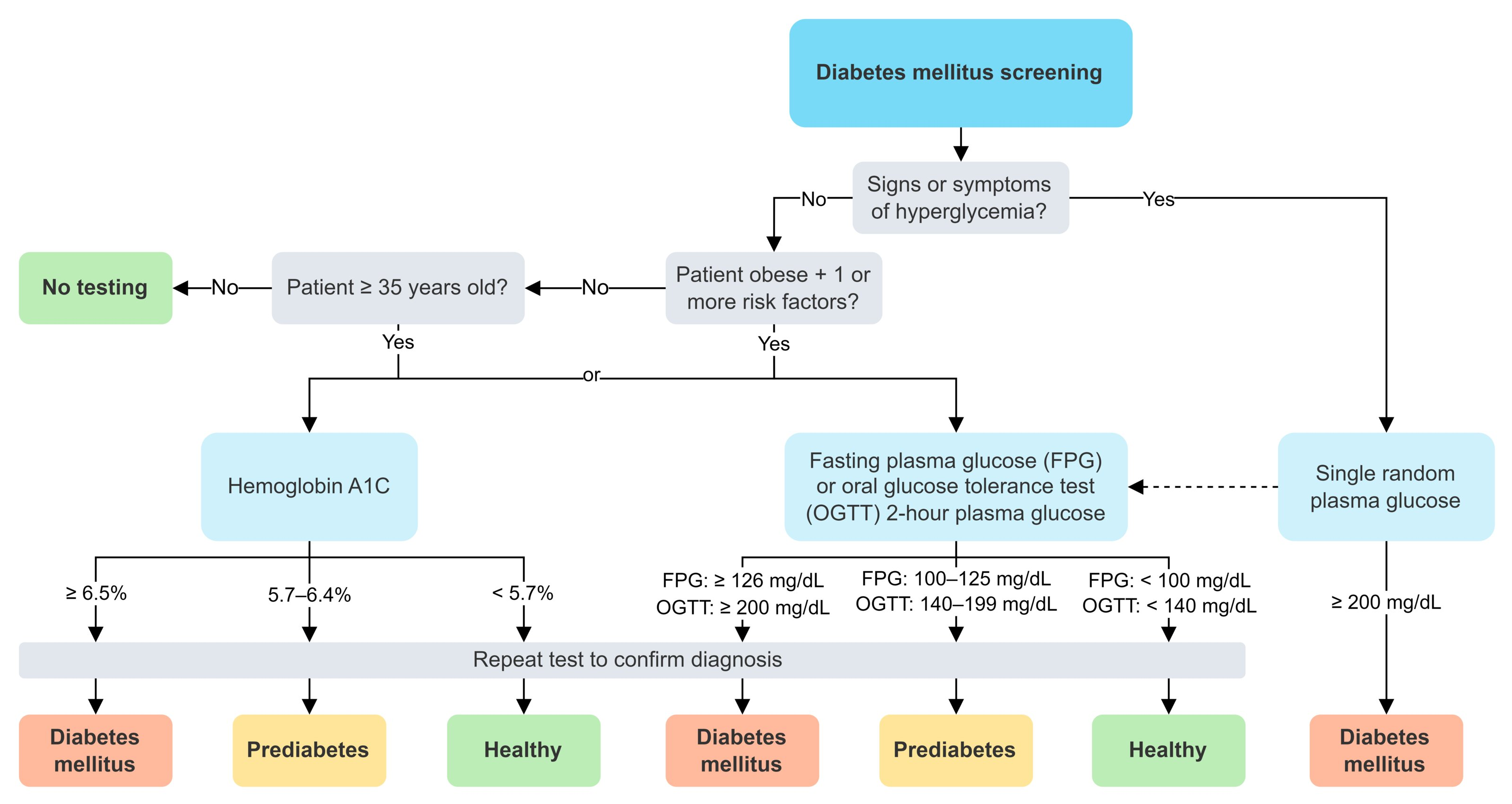
Mnemonic
Remember HbA1c as 4,5,6,7
Diagnostic criteria for diabetes mellitus
Hyperglycemia tests
Tip
In the initial stages, glucose is typically normal when fasting (ie, able to produce some insulin) but elevated after a glycemic load.
- Hemoglobin A1C (HbA1c or A1C): glycated hemoglobin, which reflects the average blood glucose levels of the prior 8–12 weeks
- Can be measured at any time, not required to fast and the results are independent of time of day
- Results may be altered by a variety of conditions or treatments, e.g., sickle cell trait, chronic kidney disease.
- Factors resulting in a falsely high HbA1c
- Increased RBC lifespan: e.g., iron and/or vitamin B12 deficiency, splenectomy, aplastic anemia
- Assay interference: heavy alcohol use
- Factors resulting in a falsely low HbA1c
- Decreased RBC lifespan: e.g., due to acute blood loss, hemoglobinopathies such as sickle cell trait/disease, thalassemia, G6PD deficiency, cirrhosis, hemolytic anemia, splenomegaly, antiretroviral drugs
- Increased erythropoiesis: e.g., due to EPO therapy, reticulocytosis, pregnancy (second and third trimesters), iron supplementation
Additional studies
- C-peptide: can help differentiate between types of diabetes
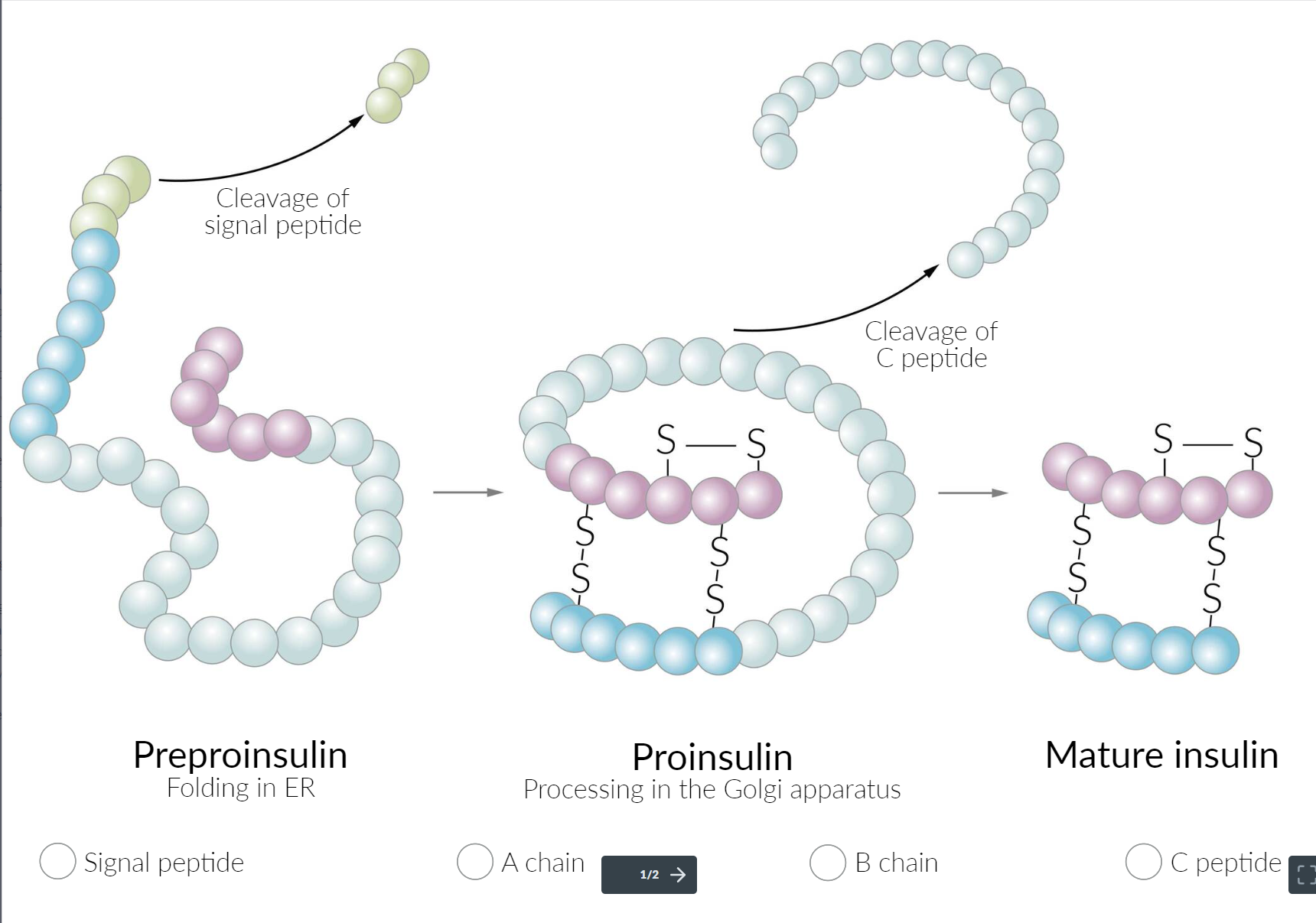
- ↑ C-peptide levels may indicate insulin resistance and hyperinsulinemia → T2DM
- ↓ C-peptide levels indicate an absolute insulin deficiency → T1DM
Treatment
T1DM
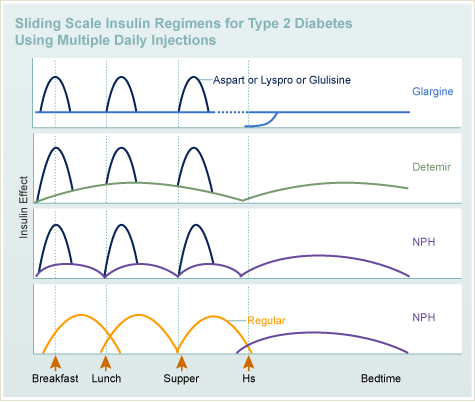
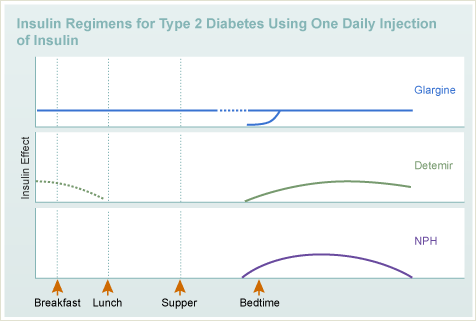
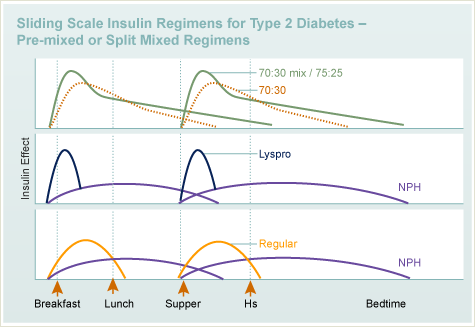
Insulin
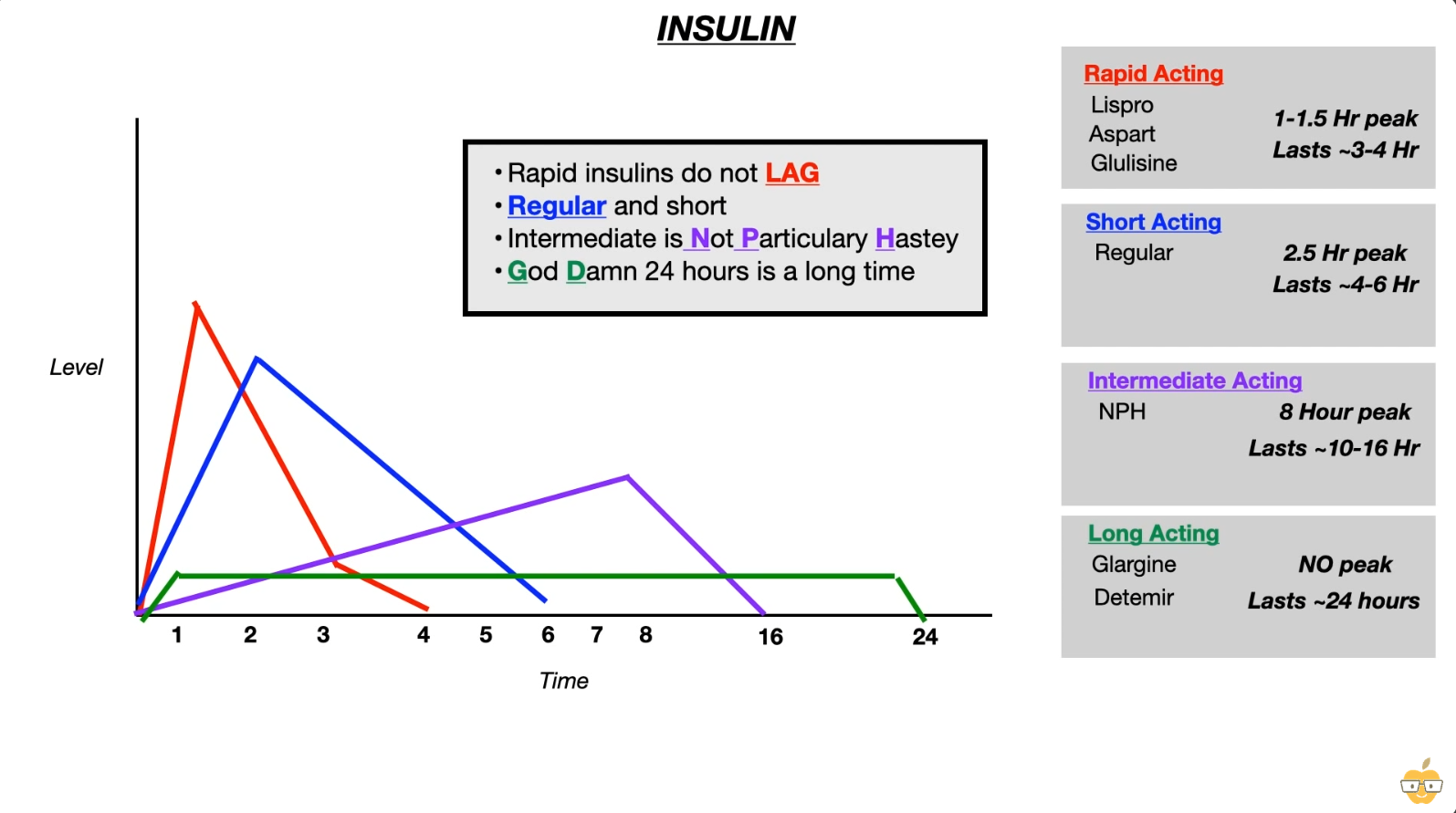
- Types
Mnemonic
- Rapid acting
- Lispro: 利索
- Aspart: A-speed
- glulisine: glu利索
- Long acting
- Glargine, detemir: large time to determine
Tip
- Rapid acting insulin has to be injected before meal, so that its peak action coincides with the rise in blood glucose levels from the food.
- Glargine is a long-acting insulin analogue that forms insoluble complexes. This leads to the formation of microprecipitates at the injection site that then slowly dissolve and are released into the circulation throughout the day.
- Regulation
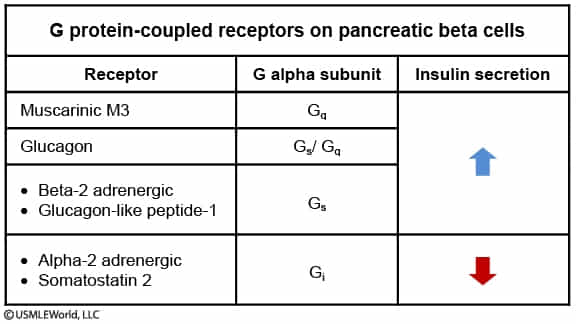
- Insulin release is primarily stimulated by the parasympathetic nervous system.
- Parasympathetic stimulation of muscarinic M3 receptors promotes insulin secretion and is induced by the smell and/or sight of food.
- In contrast, sympathetic stimulation is more complex, since both alpha-2 and beta-2 adrenergic receptors are present on pancreatic beta cells and exert opposite effects.
- Alpha-2 receptor activation (dominant) inhibits insulin release during stress to maintain high glucose levels for vital tissues (brain, heart, etc.).
- Beta-2 receptor activation modestly promotes insulin secretion, fine-tuning glucose regulation and preventing excessive hyperglycemia under moderate stress.
- However, the alpha-2-mediated inhibitory effect is predominant, causing sympathetic stimulation to lead to overall inhibition of insulin secretion.
- Alpha-2 inhibits NA reuptake
- Adverse effects
- Weight gain
- Due to physiologic factors (increased glucose uptake and lipid synthesis) and behavioral factors (less dietary adherence and eating snacks when hypoglycemia)
- Should review for changes in diet
- Weight gain
- Regimens
T2DM
Weight-favorable?
- Weight-loss medications
- GLP-1 agonist
- Decreases gastric emptying
- Increases satiety (hypothalamic effect)
- SGLT-2 inhibitor
- Increases glucosuria
- Weight-neutral medications
- DPP-4 inhibitor
- Inhibits metabolism of GLP-1
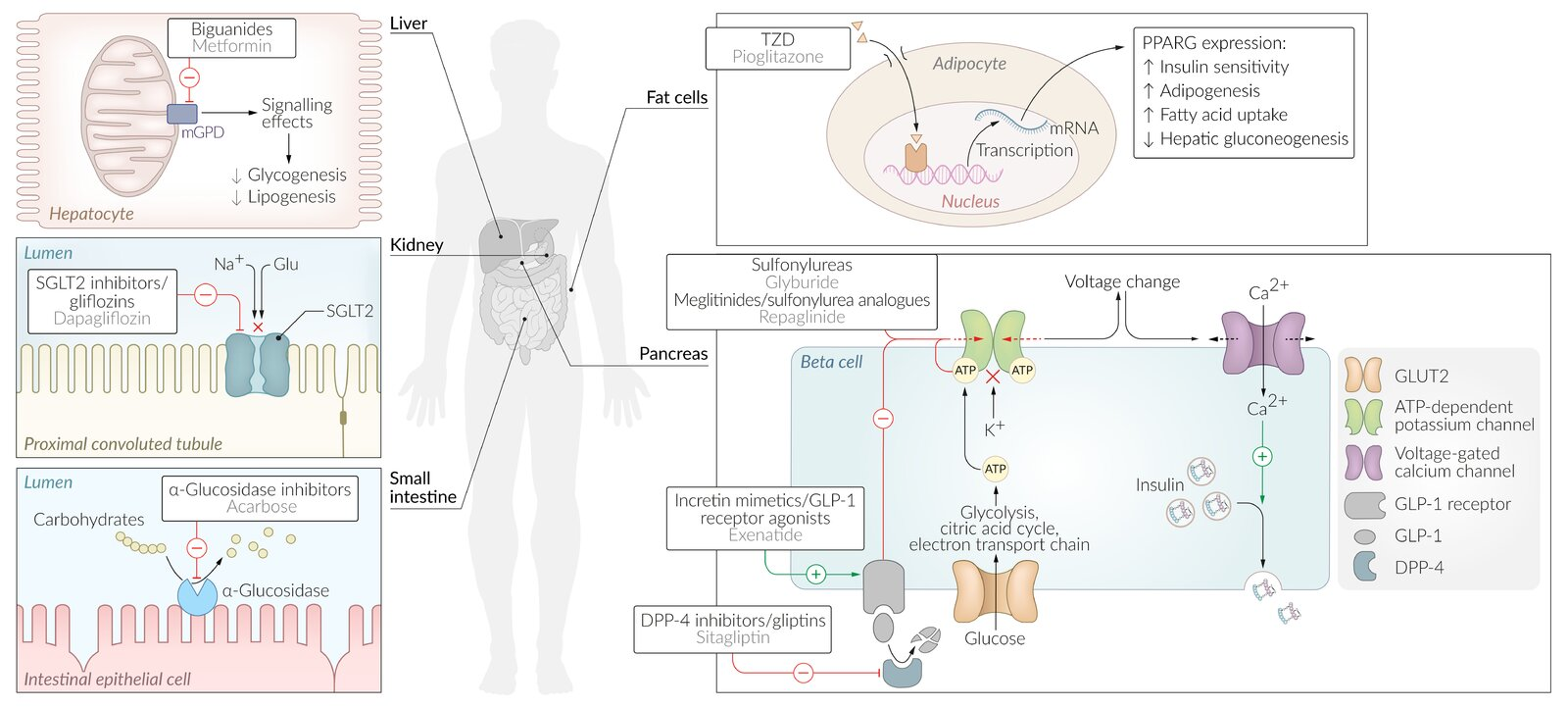
- Metformin
- Suppresses hepatic gluconeogenesis
- Decreases gastrointestinal glucose absorption
- Metformin
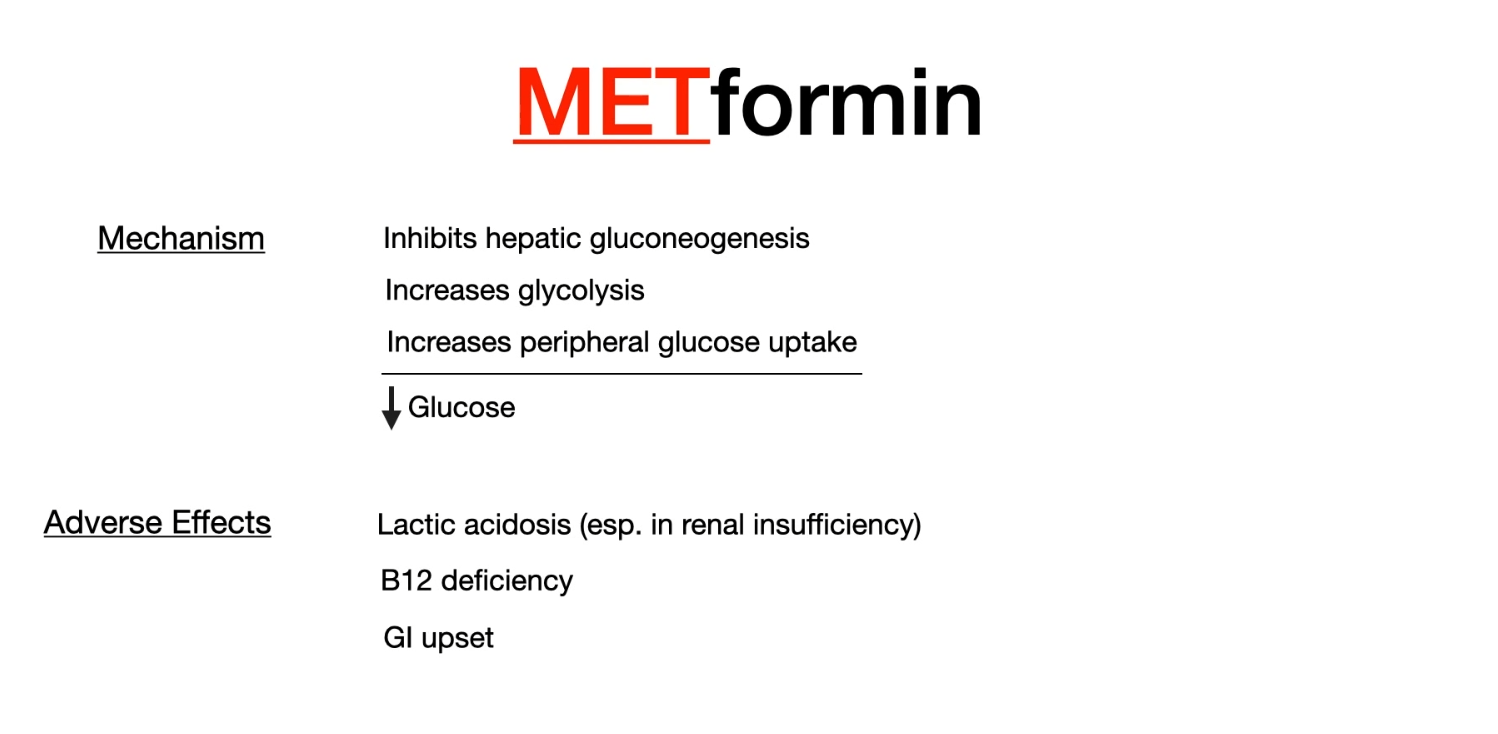
- Contraindications
- Renal failure, heart failure (NYHA III and IV), respiratory failure, shock, sepsis
- Decreased renal blood flow, cannot be excreted
- Renal failure, heart failure (NYHA III and IV), respiratory failure, shock, sepsis
- Contraindications
Mnemonic
Metformin accumulation will cause metabolic acidosis (lactic acidosis), so stop it before contrast CT scan.
- Sulfonylureas

- Close K+ channels in pancreatic B cell membrane → cell depolarizes → insulin release via ↑ Ca2+ influx.
- Side effects
- Life-threatening hypoglycemia
- Due to excessive insulin secretion, which occurs independent of blood glucose concentrations (ie, even when blood glucose concentrations are low).
- Increased risk under the following circumstances:
- Simultaneous intake of CYP2C9 inhibitors (e.g., amiodarone, trimethoprim, fluconazole)
- Patients with renal failure
- Because sulfonylureas are excreted via the kidneys, renal failure can result in accumulation. Hypoglycemia due to accumulation of long-acting glyburide may persist for several days, requiring extensive glucose substitution.
- Decreased carbohydrate intake (diets or periods of fasting)
- Elevated glucose utilization (e.g., unaccustomed physical activity)
- Sulfonylurea overdose
- Alcohol intolerance (first-generation agents): disulfiram-like reaction
- Weight gain
- Hematological changes: granulocytopenia, hemolytic anemia
- Life-threatening hypoglycemia
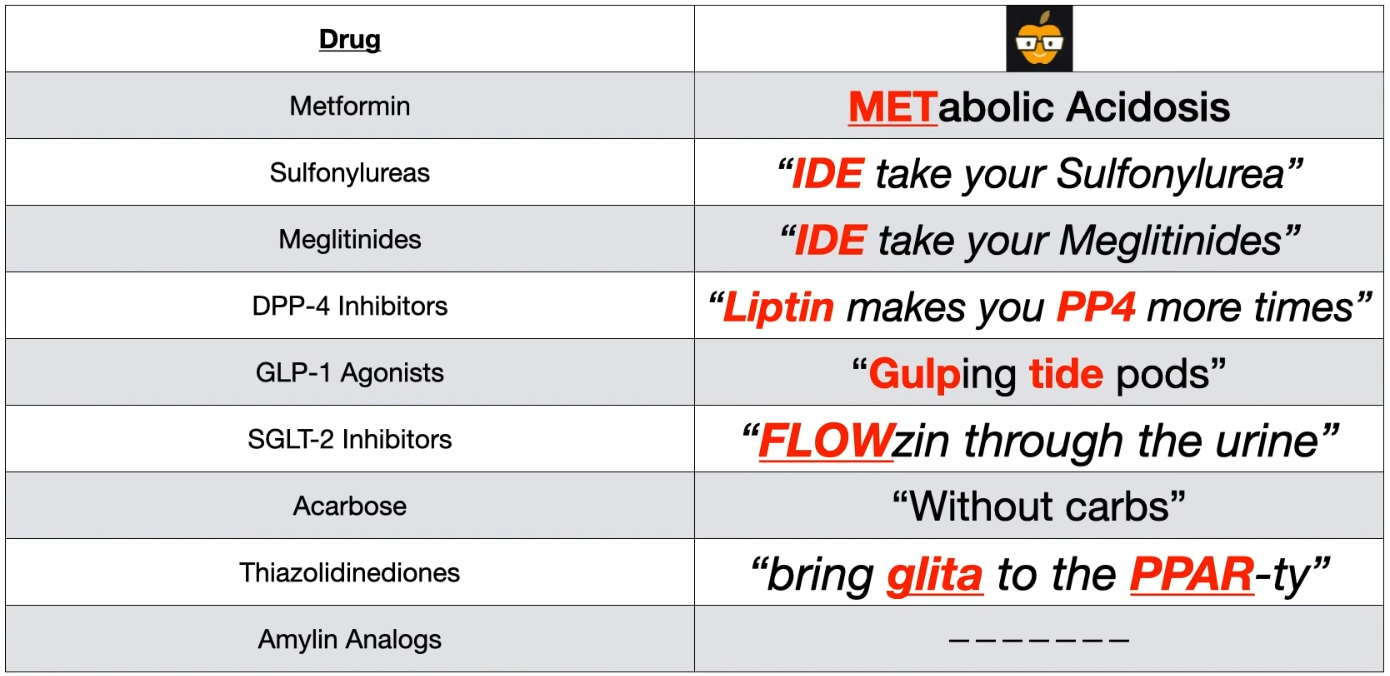
Mnemonic
G (Gee), IDE take your sulfonylurea. The G is because all sulfonylureas start with G. This helps to separate the sulfonylureas from the meglitinides since they also end with IDE
- Meglitinides
- Same with Sulfonylureas

- Same with Sulfonylureas
- DPP-4 inhibitor and GLP-1 analogs
- Although Glucagon-like peptide-1 (GLP-1) and glucagon share similar structure, they perform opposite functions.
- Glucagon-like peptide-1 (GLP-1) is an incretin hormone, meaning it’s produced in the gut after eating and signals the pancreas to increase insulin secretion.
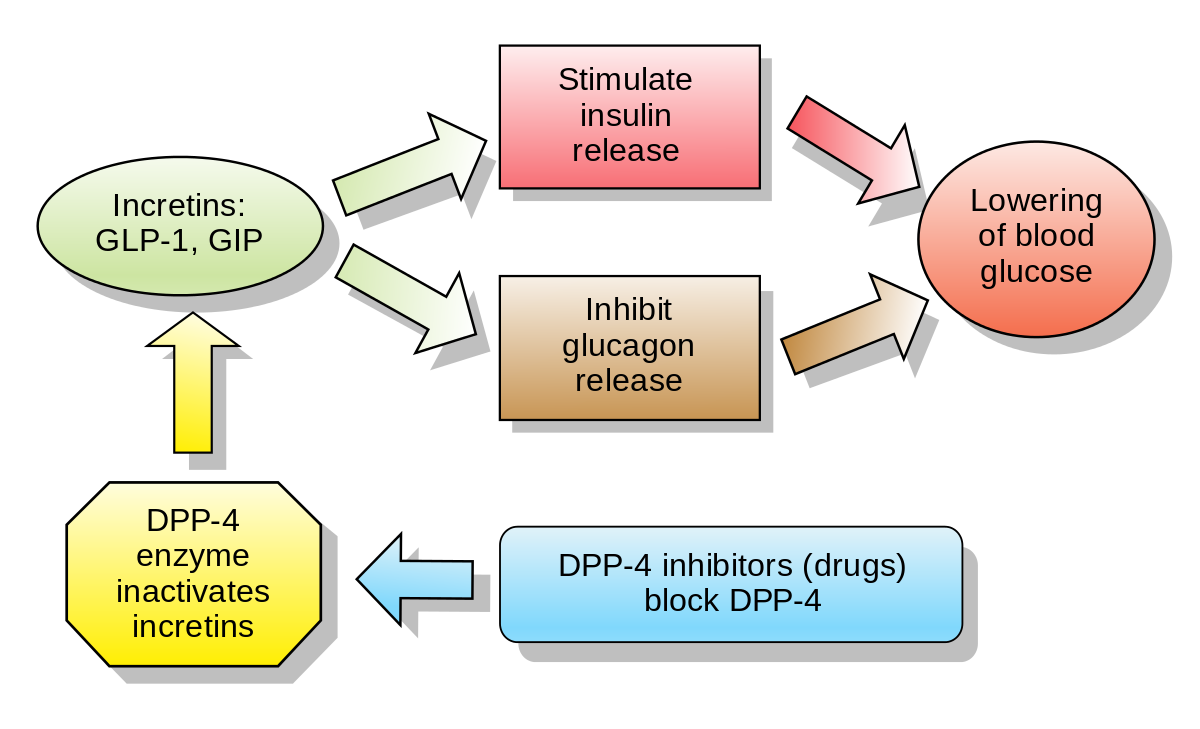
- Glucagon-like peptide-1 (GLP-1) is an incretin hormone, meaning it’s produced in the gut after eating and signals the pancreas to increase insulin secretion.

- Although Glucagon-like peptide-1 (GLP-1) and glucagon share similar structure, they perform opposite functions.
Mnemonic
- Gulfing down tide pods → GLP-1 agonist
- Liptin makes you pee (PP4) more times
- SGLT-2 inhibitors
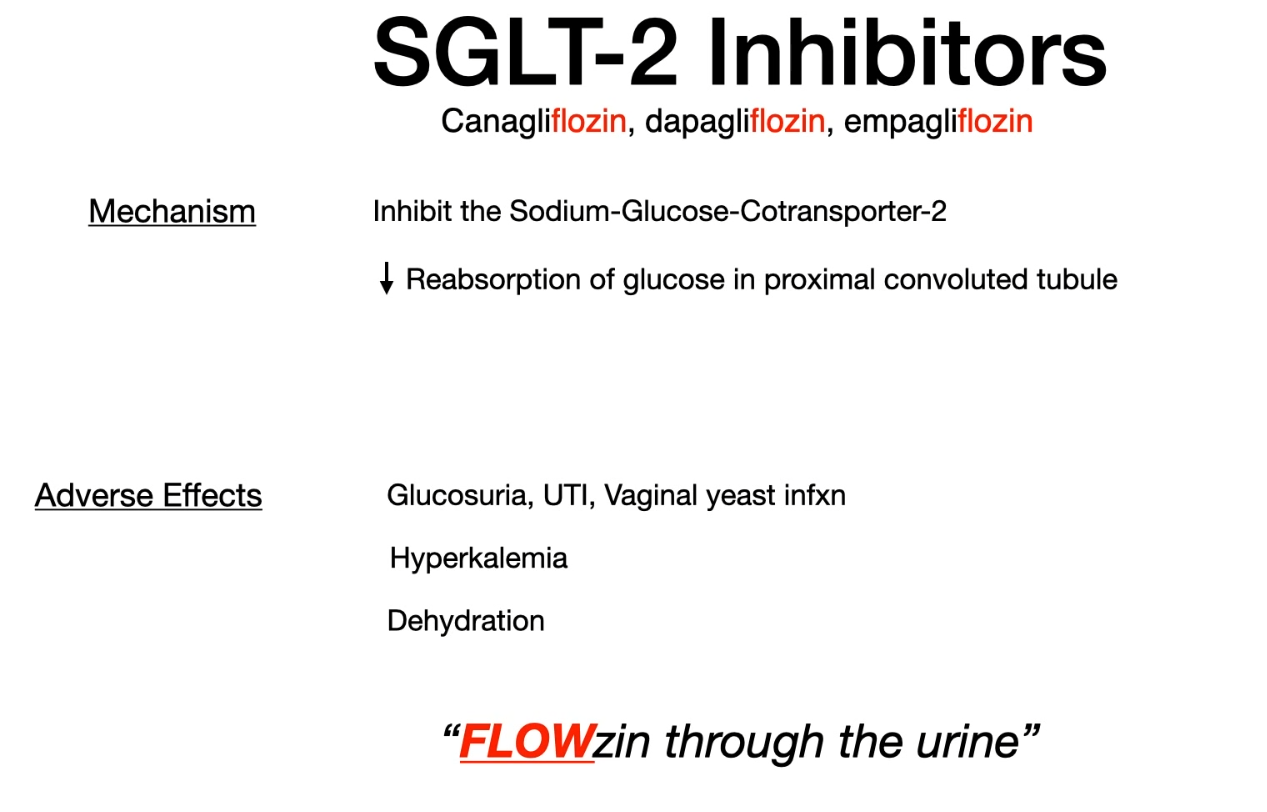
- Sodium-dependent glucose cotransporter (SGLT)
- SGLT1 is responsible for almost all sodium-dependent glucose uptake in the small intestine
- SGLT2 is responsible for almost all sodium-dependent glucose reabsorption in the proximal tubule.
- Contraindications
- Chronic kidney disease: If GFR decreases, drug efficacy decreases and adverse effects increase.
- Because SGLT-2 inhibitors rely on adequate renal glucose filtration to exert their antihyperglycemic effect, they become less effective or ineffective as renal function declines (eg, when estimated glomerular filtration rate (eGFR) falls <30-45 mL/min/1.73 m2). Therefore, checking serum creatinine (used to calculate eGFR) is recommended prior to medication initiation.
- Recurrent urinary tract infections (e.g., in patients with anatomical or functional anomalies of the urinary tract)
- Chronic kidney disease: If GFR decreases, drug efficacy decreases and adverse effects increase.
- Sodium-dependent glucose cotransporter (SGLT)
- α-glucosidase inhibitors

- Pioglitazone
- Mechanism of action: activation of the transcription factor PPARγ (peroxisome proliferator-activated receptor of gamma type in the nucleus) → ↑ transcription of genes involved in glucose and lipid metabolism → ↑ levels of adipokines such as adiponectin and insulin sensitivity → ↑ storage of fatty acids in adipocytes, ↓ products of lipid metabolism (e.g., free fatty acids) → ↓ free fatty acids in circulation → ↑ glucose utilization and ↓ hepatic glucose production
- Clinical characteristics
- Glycemic efficacy: lowers HbA1c by 1% in 3 months
- Favorable effect on lipid metabolism: ↓ triglyceride, ↓ LDL, ↑ HDL
- No risk of hypoglycemia
- Onset of action is delayed by several weeks.
- because it acts at the level of transcription
- Important side effects
- ↑ Risk of heart failure
- ↑ Risk of bone fractures (osteoporosis)
- Fluid retention and edema
- Weight gain

Mnemonic
- glita ← glitter
- Eat in the party will make you gain weight.
Tip
Fibrates activate PPAR-α. See Lipid-lowering agents > Fibrates
- Amylin analogs (Pramlintide)
- Works with insulin to ↓ glucagon release, ↓ gastric emptying.
Mnemonic
Amylin analogs (Pramlintide) works with insulin.
Screening
- All individuals ≥ 35 years of age
- Patients < 35 years of age who:
- Are overweight or obese AND have ≥ 1 additional risk factor for T2DM
- BMI of ≥ 23 kg/m2 in Asian American individuals or a BMI of ≥ 25 kg/m2 in all other individuals
- Are overweight or obese AND have ≥ 1 additional risk factor for T2DM