Tip
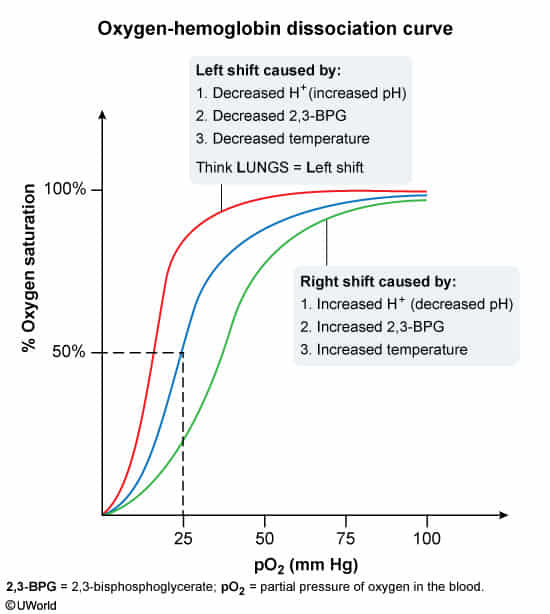
When oxygen supply is insufficient, the body initially reduces the affinity of red blood cells for oxygen, ensuring adequate oxygen supply to peripheral tissues.
- Causes of shift to the right include:
- ↑ Partial pressure of carbon dioxide (↑ PCO2)
- ↑ Body temperature (e.g., fever)
- ↑ H+ (↓ pH)
- ↑ 2,3-BPG (generated by 2,3-BPG mutase during erythrocyte glycolysis)
- Pathophysiology/Etiology
- 2,3-BPG is a three-carbon isomer of the glycolytic intermediate 1,3-BPG. It is produced in erythrocytes (RBCs) via the Rapoport-Luebering shunt, a detour from the standard glycolytic pathway.
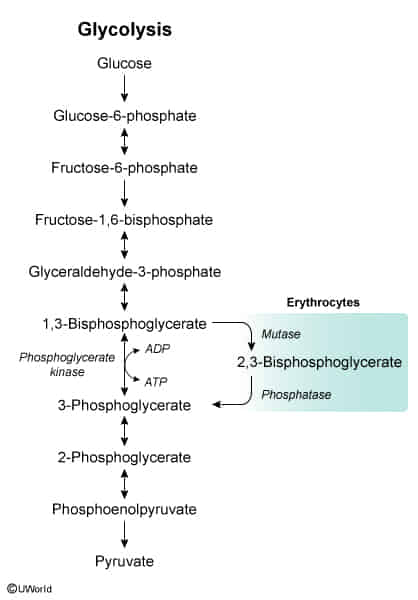
- Its primary function is to regulate the oxygen affinity of hemoglobin. By binding to the deoxygenated “T” (taut) form of hemoglobin, 2,3-BPG stabilizes it, thereby decreasing hemoglobin’s affinity for O2.
- This stabilization facilitates the unloading of oxygen to tissues.
- 2,3-BPG is a three-carbon isomer of the glycolytic intermediate 1,3-BPG. It is produced in erythrocytes (RBCs) via the Rapoport-Luebering shunt, a detour from the standard glycolytic pathway.
- Regulation of Synthesis
- Increased in states of chronic hypoxia and anemia as a compensatory mechanism. This includes conditions like high altitude, chronic lung disease, and heart failure.
- Synthesis is also stimulated by alkalosis and hyperthyroidism.
- Decreased by hypophosphatemia and acidosis. During blood storage (e.g., in acid-citrate-dextrose), 2,3-BPG levels drop significantly, causing stored blood to have a very high O2 affinity (left-shift), which temporarily impairs oxygen delivery immediately after transfusion.
- Pathophysiology/Etiology
- ↑ Exercise
- ↑ Altitude
- Causes of shift to the left include:
- ↓ Partial pressure of carbon dioxide (PCO2)
- ↓ Body temperature
- ↓ H+ (↑ pH)
- ↓ 2,3-BPG (e.g., due to mutations in the BPGM gene on chromosome 7)
- ↑ CO
- ↑ Methemoglobin
- ↑ Fetal hemoglobin (HbF)