Alcohol metabolism
Key Point
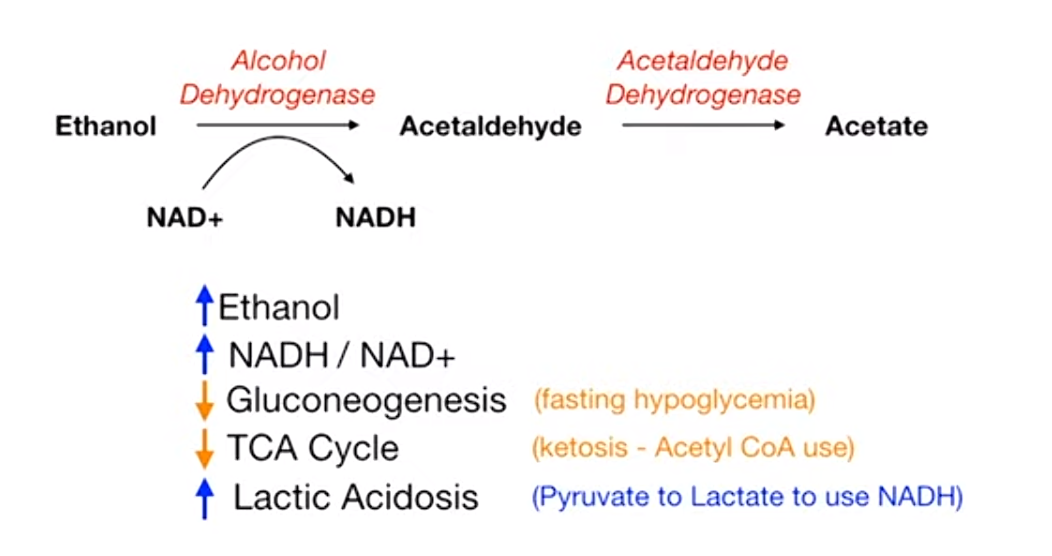
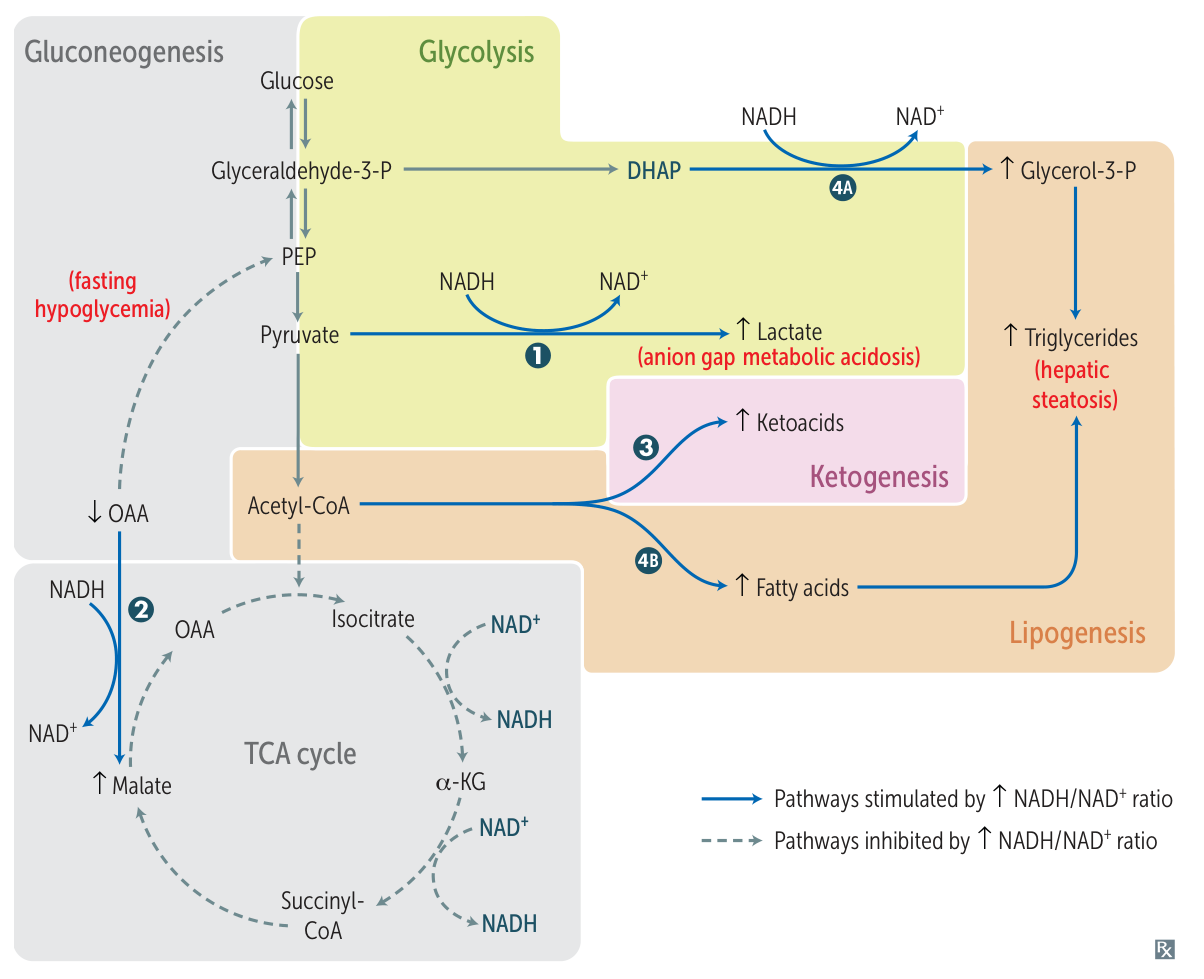
Ethanol metabolism increase NADH/NAD+, which further downregulate gluconeogenesis and TCA cycle, and increase lactate.
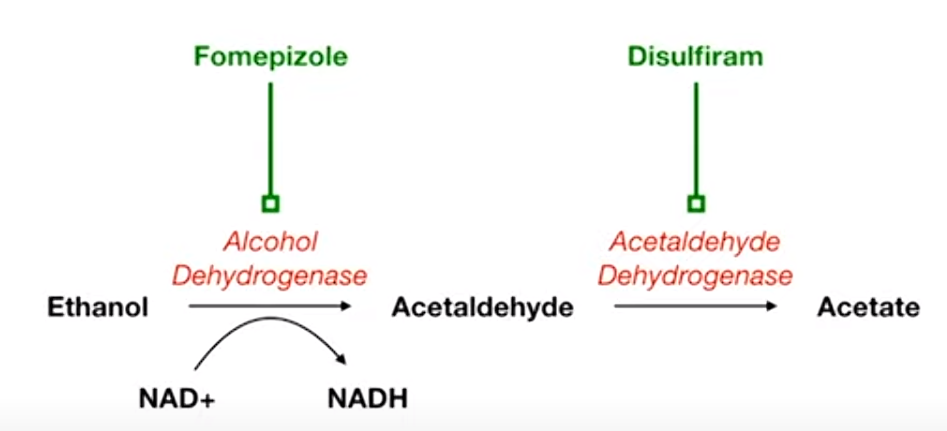
Mnemonic
- Fomepizole: think it as Foam-epizole, which can turn the beer to a lot of foam and less liquid (dehydro).
- Used for alcohol intoxication
- Disulfiram: diSUFFERam. It can increase acetaldehyde and make people sick and dizzy.
- Used in alcohol use disorder, as 2nd line treatment
Treatment
Pharmacotherapy
First-line options
- Naltrexone and acamprosate are preferred.
- Naltrexone: central μ-opioid receptor antagonist
- Acamprosate: may modulate central glutamate receptors
- Both medications reduce cravings and can be used for patients who:
- Continue to drink alcohol
- Use other substances recreationally
Alternative options
- Disulfiram
- Topiramate
- Gabapentin
Complications
Multisystem complications
- Cytochrome P450 induction
- Vitamin deficiency
- Vitamin B1 deficiency (thiamine deficiency): Wernicke-Korsakoff syndrome
- Vitamin B6 deficiency: peripheral neuropathy
- Vitamin B9 deficiency (folate deficiency): megaloblastic anemia
- Depletion would take place over weeks
- Vitamin B12 deficiency: subacute combined degeneration of spinal cord (funicular myelosis), megaloblastic anemia
- Depletion would take place over years
- Electrolyte abnormalities: Hyponatremia, Hypokalemia, Hypocalcemia
- Often caused by complications such as malnutrition, vomiting, or diarrhea
Alcohol intoxication
Pathophysiology
- Mechanism
- GABA-A receptor agonist → Chronic use causes downregulation of GABA-A receptors → decreased GABA-A activity when cessation
- NMDA glutamate antagonist → chronic use causes upregulation of NMDA receptors → increased glutamate NMDA activity when cessation
- Absorption: The majority of alcohol consumed is absorbed by the proximal small intestine; only a small amount of alcohol is absorbed by the oral, esophageal, and/or gastric mucosa.
Clinical features
Altered consciousness, cognition, perception, judgment, affect, and/or behavior.
Diagnostics
- Routine laboratory studies that support the diagnosis include:
- Elevated osmolar gap
- The difference between the measured serum osmolarity and the calculated serum osmolarity. Normally, the calculated serum osmolarity is primarily determined by circulating levels of sodium salts (chloride and bicarbonate), glucose, and urea. An increased serum osmolar gap occurs when other solutes (e.g., ethanol, methanol, propylene glycol) are present in high enough concentrations to increase the measured osmolarity by more than 10 mOsmol/L.
- Anion gap metabolic acidosis (e.g., ↓ pH, ↓ HCO3-)
- Elevated osmolar gap
Treatment
Alcohol dehydrogenase inhibitors
- Goal: to prevent the conversion of methanol or ethylene glycol to toxic metabolites (e.g., formic acid, glycolic acid, oxalic acid) by competitively inhibiting alcohol dehydrogenase
- Options
- Fomepizole (first-line): a competitive alcohol dehydrogenase inhibitor; safer and easier to administer than ethanol
- Ethanol (second-line)