The nephron
Tip
- Iso-osmotic reabsorption: The proximal tubule reabsorbs sodium and water in equal proportions. This means that while a large amount of sodium is reabsorbed, the concentration in the tubular fluid remains relatively constant.
- Active and passive transport: Sodium reabsorption is driven by active transport mechanisms, primarily the Na+/K+ ATPase pump. This creates an electrochemical gradient that drives the reabsorption of other solutes, including water. Potassium is reabsorbed both passively and actively.
- Regulation: Hormonal mechanisms, such as aldosterone, can fine-tune sodium reabsorption in later parts of the nephron (distal tubule and collecting duct), but in the proximal tubule, the focus is on bulk reabsorption.
- Renal pH: lowest at collecting duct
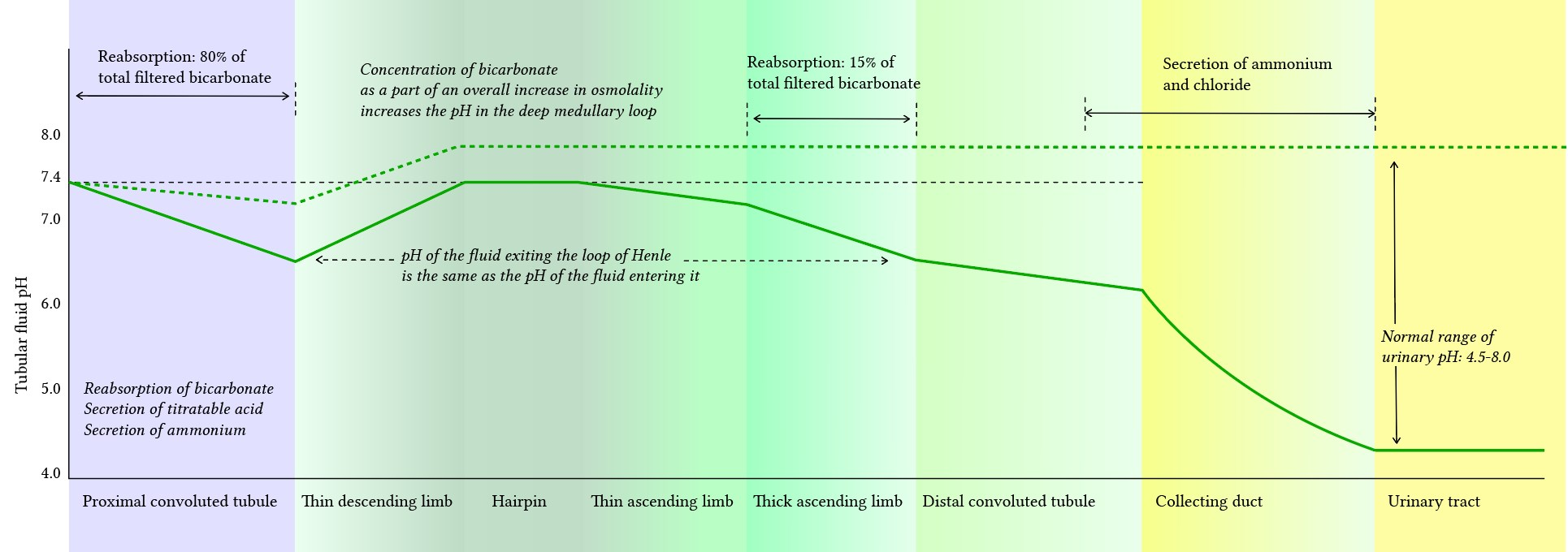
Proximal convoluted tubule (PCT)
- Bicarbonate
- Don’t change H+, only absorb HCO3-
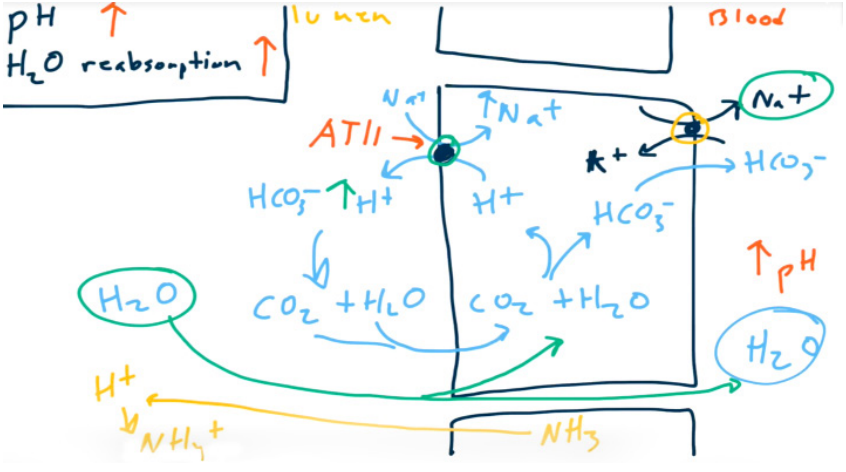
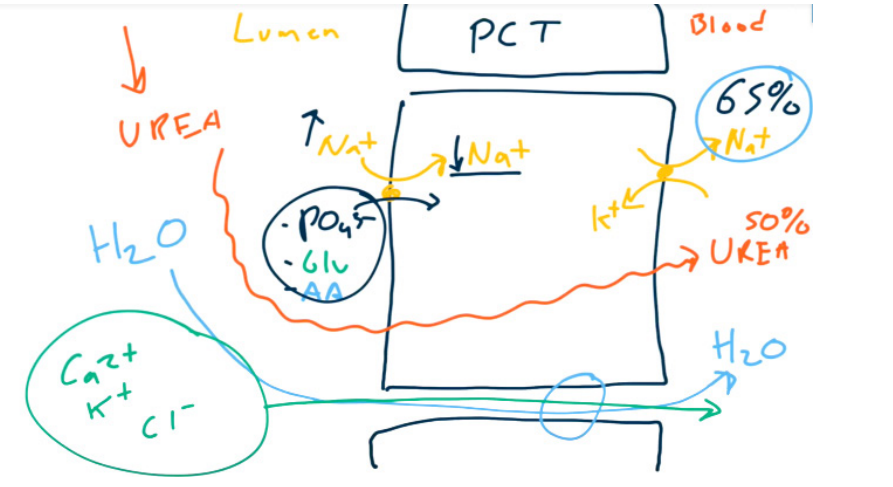
- Absorb Na+ to absorb H2O. 65% of sodium and water are absorbed in PCT.
- Angiotensin II increases Na+, HCO3-, and H2O reabsorption via Na+/H+ exchanger stimulation (allows for contraction alkalosis).
- Don’t change H+, only absorb HCO3-
- Parathormone (PTH) decreases PO43- reabsorption via Na+-PO43- cotransporter inhibition.
- Brush border resorption of most of the ultrafiltrate
- Glucose (via SGLT2)
- Amino acids
- Uric acid
- Na+, Cl-, K+, HCO3-, PO43-, and H2O (All together with Na+ !!!)
Thick ascending loop of Henle
- ADH stimulates the activity of Na+-K+-2Cl- cotransporter. Increased NKCC2 activity aids in water reabsorption in the collecting duct through aquaporin 2 channels by creating a hypo-osmotic filtrate.
Distal convoluted tubule (DCT)
- Magnesium and Calcium are reabsorbed paracellularly.
- Ca2+ is reabsorbed using Ca2+ channels on the luminal surface and Na+/Ca2+ antiporters (exchangers) on the basolateral surface.
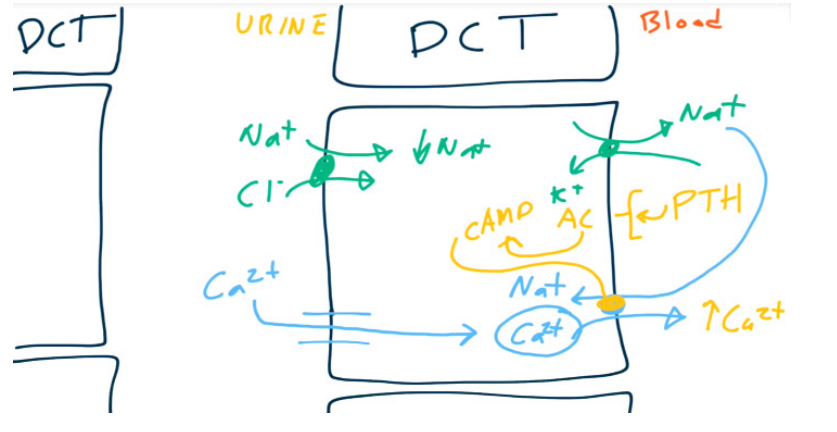
- PTH up-regulates the Ca2+/Na+ antiporter resulting in increased reabsorption of Ca2+.
Collecting duct
- Principal cells
- When acted upon by ADH, principal cells will increase the number of aquaporins (water channels) on the luminal membrane → increased H2O reabsorption.
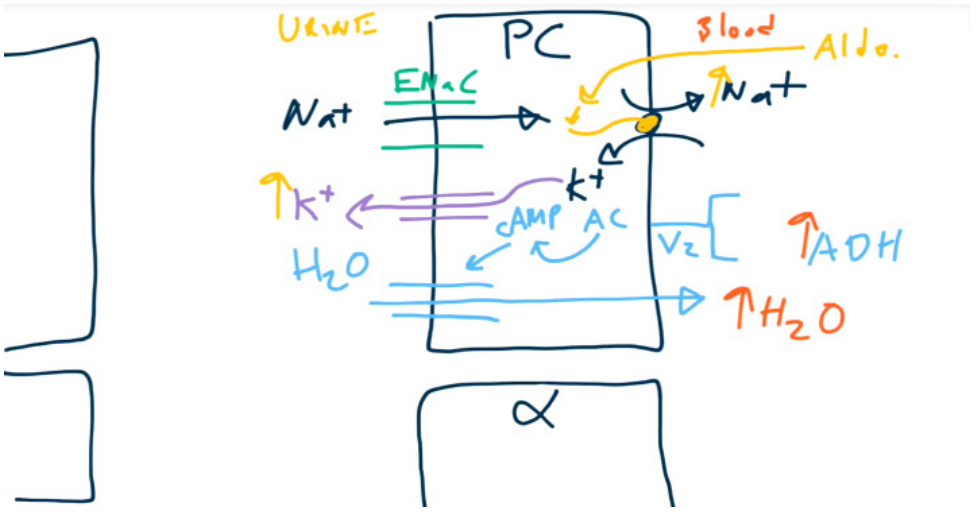
- When acted upon by aldosterone, principal cells increase activity of Na+/K+ exchangers → increased Na+ reabsorption and increased K+ secretion.
- Water follows sodium through the water channels. So aldosterone won’t affect the urine osmolality.
- When acted upon by ADH, principal cells will increase the number of aquaporins (water channels) on the luminal membrane → increased H2O reabsorption.
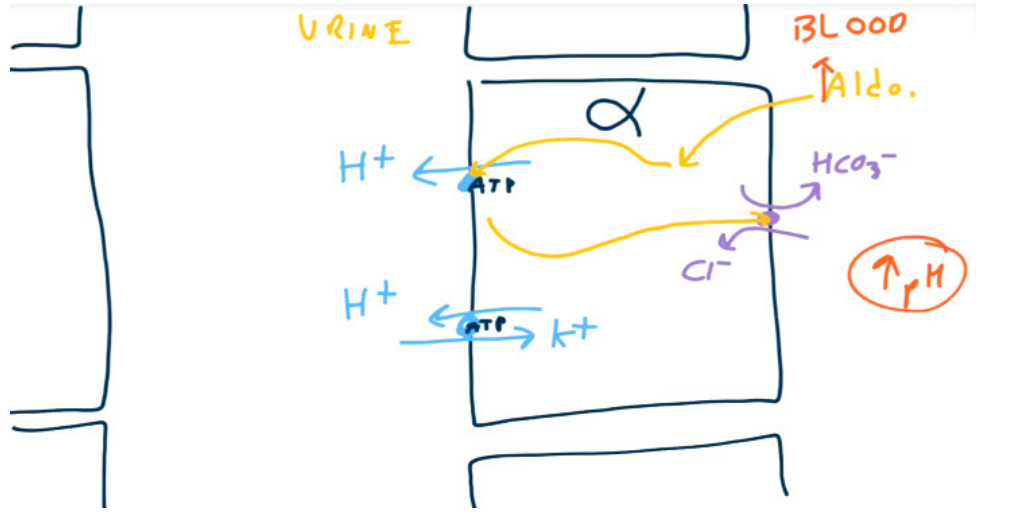
- Alpha-intercalated cells
- Have a K+/H+ exchanger on the luminal surface to secrete H+ and reabsorb K+
- Have a H+-ATPase which actively secretes H+. On the basolateral surface, the cells have a HCO3 -/Cl- exchanger to reabsorb HCO3 -.
- Aldosterone upregulates H+ secretion via H+-ATPase. Increased H+ secretion will lead to increased HCO3- reabsorption.
- ADH also stimulates reabsorption of urea in collecting ducts to maximize corticopapillary osmotic gradient. So increased BUN-creatinine ratio in hypovolemia.
- Urea passively diffuses from the interstitium into the loop of Henle, increasing the luminal concentration of urea.
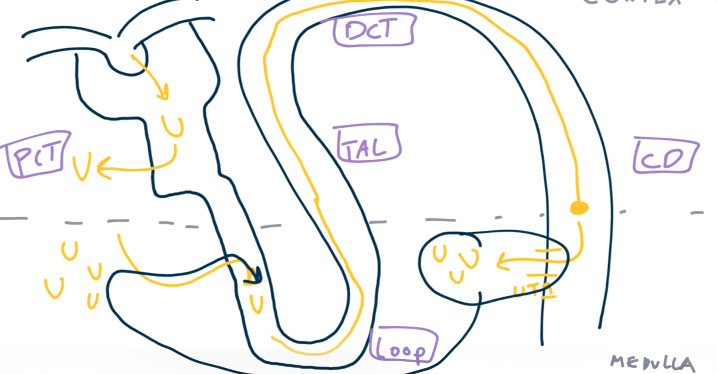
- Urea passively diffuses from the interstitium into the loop of Henle, increasing the luminal concentration of urea.
Renal blood flow
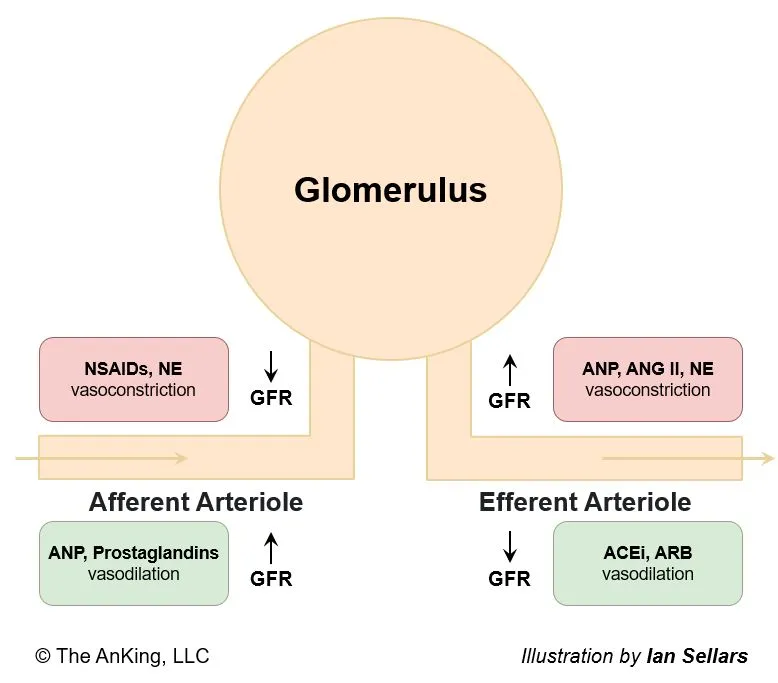
Mnemonic
Physiological substances all increase GFR, while artificial medications all reduce GFR.
Tubuloglomerular feedback
- Description: feedback system between the tubules and glomeruli that adjusts the GFR according to the resorption capacity of the tubules
- Mechanism: macula densa (of the juxtaglomerular apparatus) senses alterations in the NaCl concentration in the DCT
- Hypotonic urine (↓ intraluminal Cl- concentration) → vasodilation of afferent arterioles → ↑ GFR → ↑ Cl- intraluminal concentration → ↑ RBF
- Hypertonic urine (↑ intraluminal Cl- concentration) → adenosine secretion → vasoconstriction of afferent arterioles → ↓ capillary pressure → ↓ GFR → ↓ intraluminal Cl- concentration → ↓ RBF
Juxtaglomerular complex
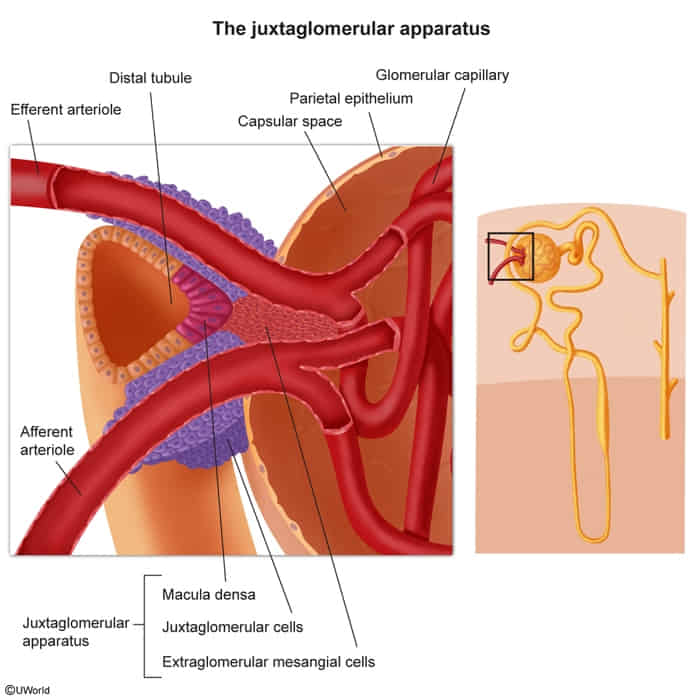
- Juxtaglomerular cells
- Modified smooth muscle cells located in the afferent arterioles
- Function: renin synthesis
- Macula densa
- Composed of tall cuboidal cells located at the distal end of the thick ascending loop of Henle
- Monitors the NaCl concentration within the lumen of the DCT
- Hypoosmolar urine triggers the release of renin → vasoconstriction of the efferent arteriole → increase in GFR
- Hyperosmolar urine triggers the release of adenosine → vasoconstriction of the afferent arteriole → decrease in GFR
- Extraglomerular mesangial cells
- Play a role in autoregulation of blood flow to the kidney (exact functioning is not entirely understood)
- Form a network of cells, connecting the sensory cells of the macula densa with juxtaglomerular effector cells
- May also signal to contractile glomerular mesangial cells and, thereby, directly affect vasoconstriction
Measurement of renal function
Filtration fraction
- Description: the fraction of the renal plasma flow (RPF) that is filtered from the capillaries into the Bowman space
- Mechanism
- FF = GFR/RPF (i.e., FF = CInulin/CPAH)
- GFR can be calculated using the inulin or creatinine clearance, as these substances are freely filtered at the glomerulus and have relatively insignificant tubular reabsorption or secretion. RPF can be determined using the para-aminohippuric acid (PAH) clearance as almost all the PAH entering the kidneys is excreted in the urine (mostly via tubular secretion).
- CInulin = inulin clearance
- CPAH = PAH clearance
- Normal: 20%
- Regulated via:
- Prostaglandins → dilation of afferent arterioles → ↑ GFR and ↑ RPF (FF unchanged)
- Angiotensin II → constriction of efferent arterioles → ↑ GFR and ↓ RPF → ↑ FF
- FF = GFR/RPF (i.e., FF = CInulin/CPAH)
Renal clearance
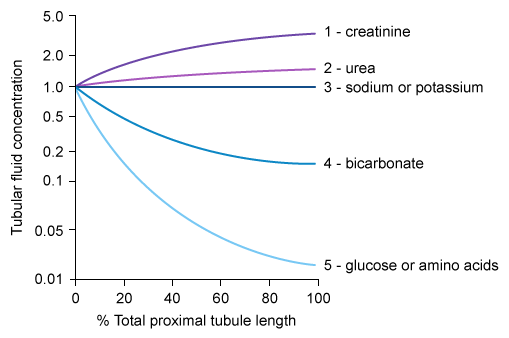
Relative solute concentrations along proximal convoluted tubules
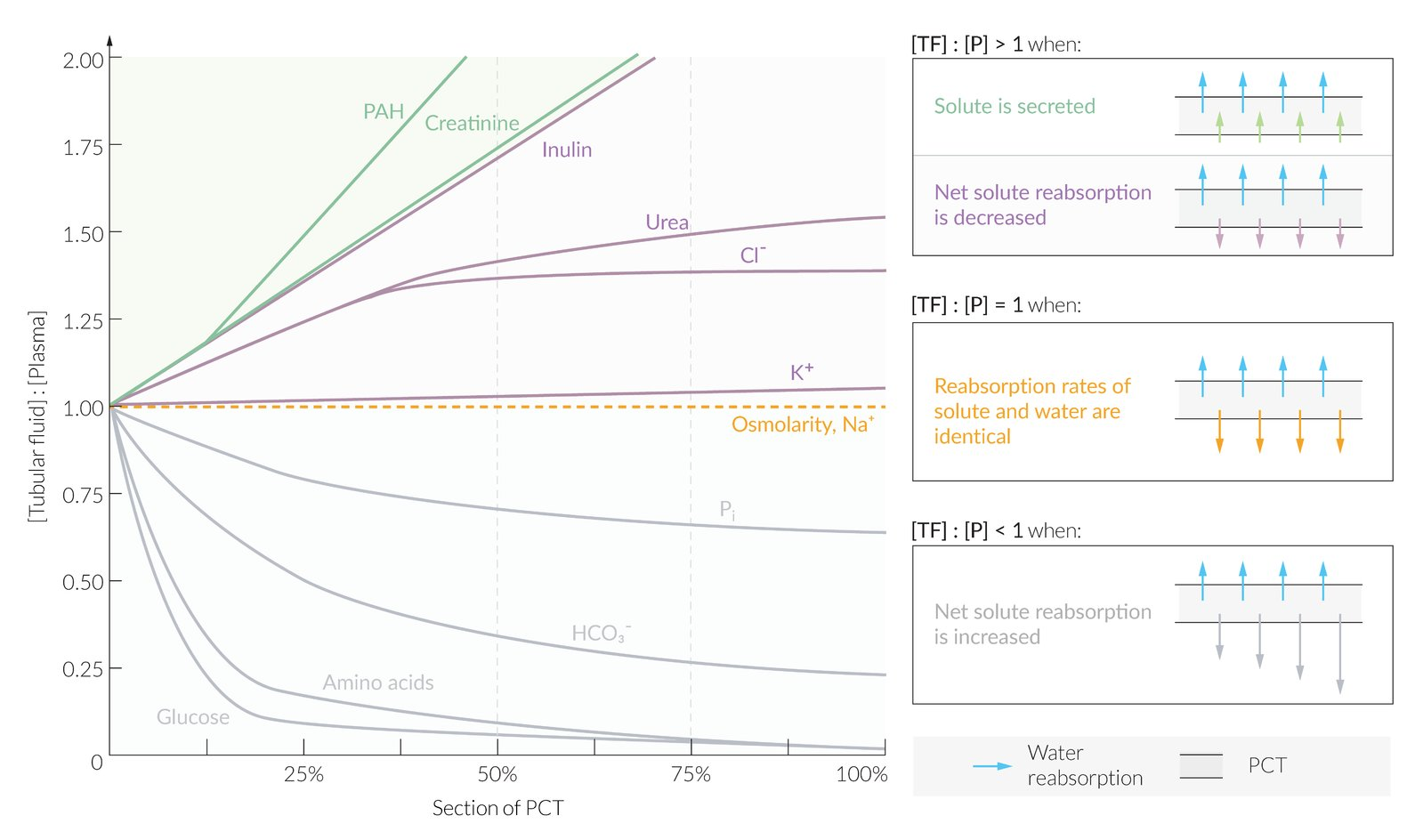
- Mechanism
- Water is absorbed along the PCT along with other solutes (e.g., creatinine, electrolytes, glucose)
- At the beginning of the PCT, the concentration of all solutes within the glomerularly filtered tubular fluid (TF) is equivalent to the plasma concentration (P).
- Compared to water, solutes can be reabsorbed along the PCT:
- At the same rate → no change in tubular fluid concentration compared to plasma concentration (TF/P = 1)
- At a lower rate → increased tubular fluid concentration compared to plasma concentration (TF/P > 1)
- At a higher rate → decreased tubular fluid concentration compared to plasma concentration (TF/P < 1)
- Specific solutes
- Sodium (Na+): reabsorbed at the same rate as water throughout the PCT → (TF/P)Sodium = 1
- Inulin
- Not reabsorbed, nor secreted along the PCT → (TF/P)Inulin > 1
- Inulin is unique in that its amount does not change along the PCT but its tubular concentration is determined solely by water reabsorption.
- PAH and creatinine: net tubular secretion along the PCT → (TF/P)PAH/Creat. /Creat. > (TF/P)Inulin > 1
- Chloride (Cl‑): (TF/P)Chloride > 1 throughout the PCT
- Initially reabsorbed at a slower rate than water and sodium → (TF/P)Chloride > 1 and rising
- More distally in the PCT, reabsorbed at the same rate as water and sodium → still (TF/P)Chloride > 1 but no longer increasing (plateaued)
- Glucose: reabsorbed at a higher rate than water → (TF/P)Glucose < 1
Kidney embryology
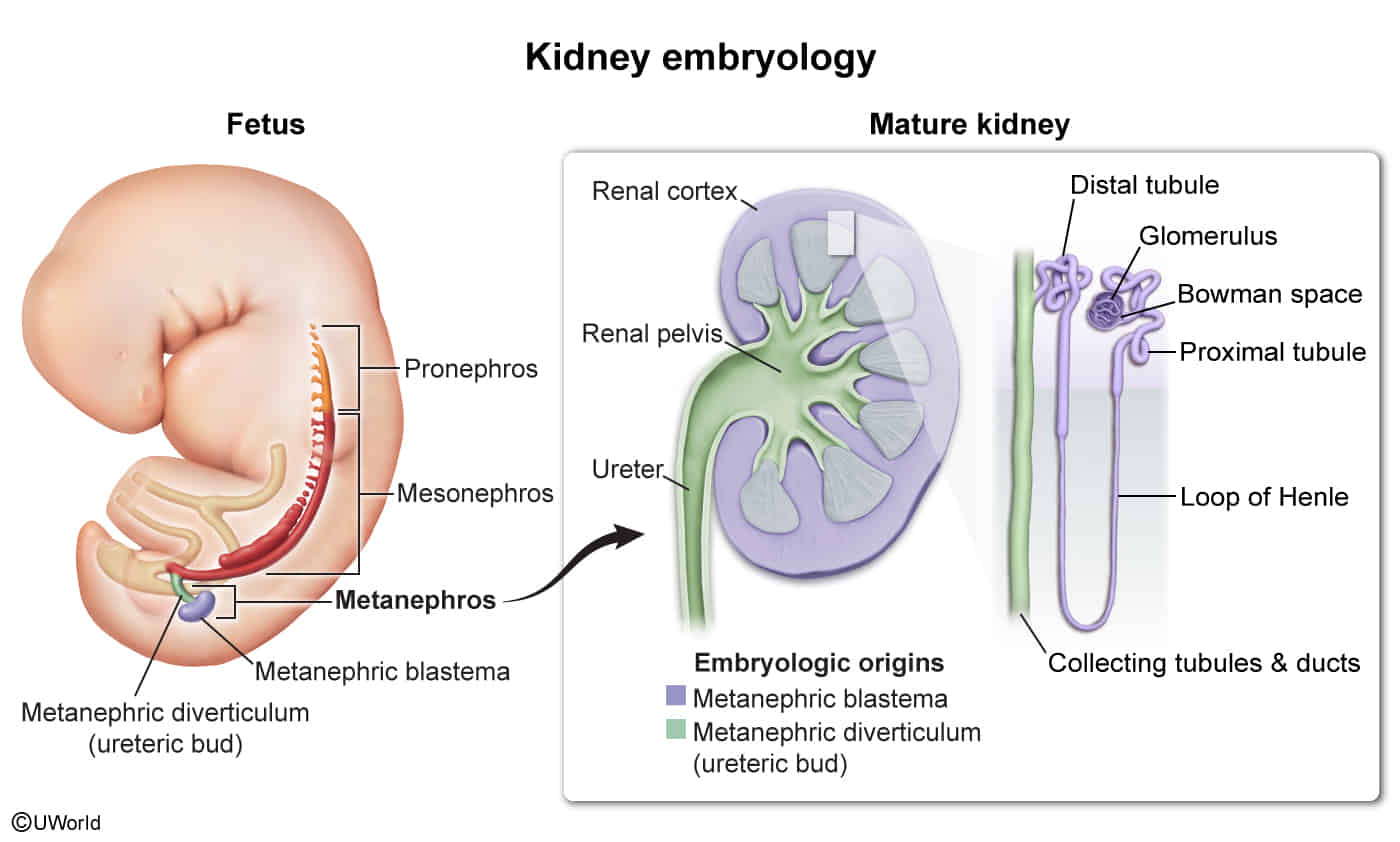
- Metanephros: the third embryonic excretory organ, developing caudally to the mesonephros and persisting as the permanent kidney
- Arises during the 5th week of embryonic development
- Canalization is complete by the 10th week of embryonic development.
- Maturing of the kidneys continues until week 35–36 of embryonic development.
- Components
- Ureteric bud (metanephric diverticulum)
- Originates from the caudal end of the mesonephric duct
- Differentiates into the collecting duct system: ureter, renal pelvis, major calyces, minor calyces, and collecting ducts
- Ureteropelvic junction: junction between ureter and renal pelvis
- Canalizes last and represents the most common site of prenatal urinary tract obstruction
- Obstruction of the ureteropelvic junction may cause prenatal hydronephrosis, which can be discovered during prenatal ultrasound screenings.
- Metanephric blastema (metanephric mesenchyme)
- Differentiation stimulated by ureteric bud signals
- Differentiates into nephrons: glomerulus, proximal convoluted tubule, loop of Henle, and distal convoluted tubule
- Ureteric bud (metanephric diverticulum)
- Congenital anomalies of the kidneys can result from, e.g., due to abnormal interaction between the ureteric bud and the metanephritic blastema.
Kidney anatomy
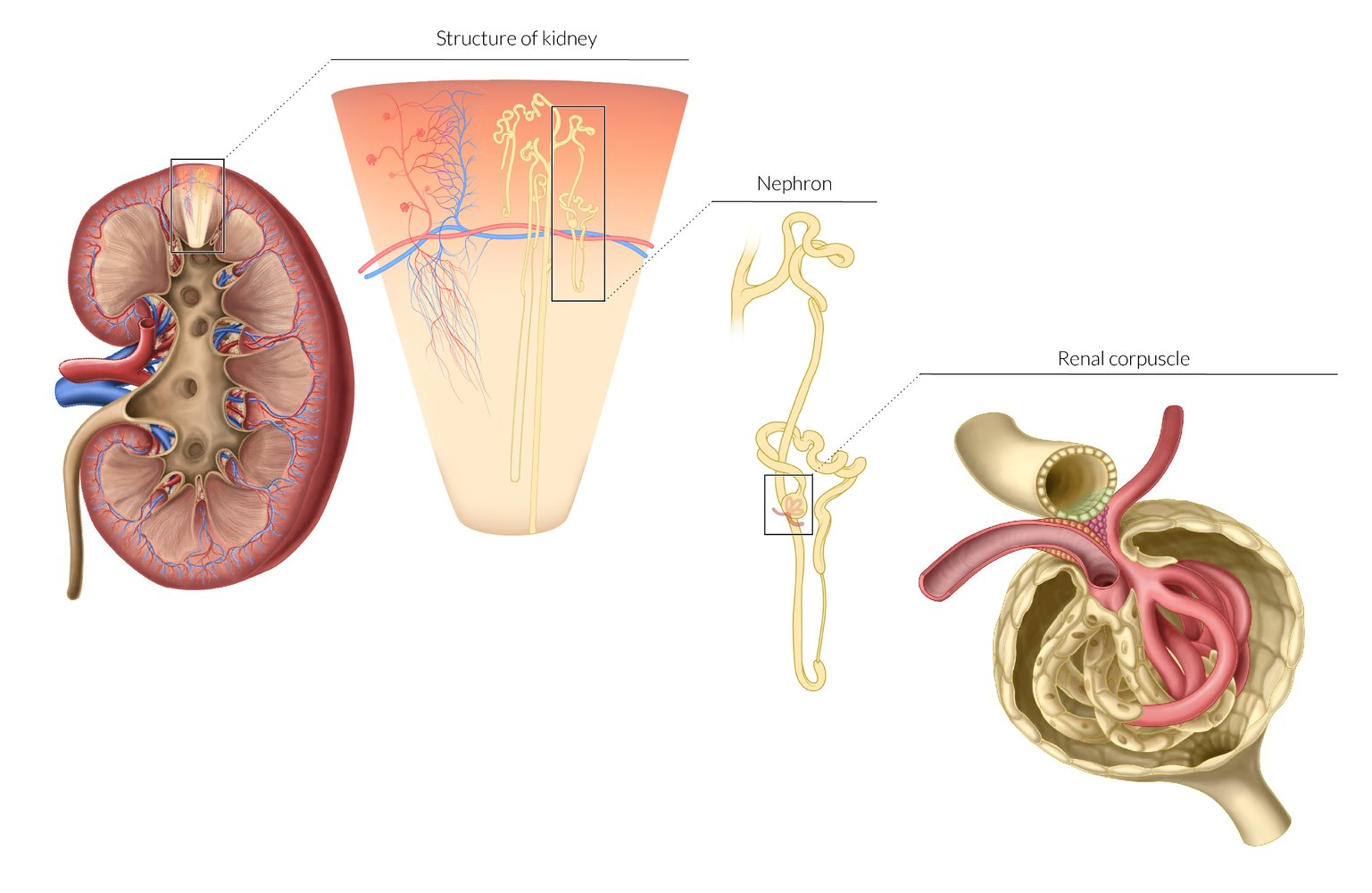
- Renal cortex
- The outermost layer of the renal parenchyma (∼ 10 mm thick)
- Surrounds the renal medulla and extends inwards (as renal columns) dividing the medulla into renal pyramids
- Contains the glomeruli, proximal convoluted tubules, distal convoluted tubules, and cortical collecting ducts
- Renal medulla
- Consists of several renal medullary pyramids separated by the renal columns
- The base of each pyramid faces the outer cortex, the apex faces the renal sinus and forms a renal papilla, which drains into a minor calyx.
- Contains the loops of Henle and collecting ducts, which merge to form the papillary ducts at the renal papillae
- Blood flow in the renal medulla is relatively low compared to that in the renal cortex.
- Facilitates the development of an osmolality gradient that allows for effective urine concentration
- Causes medullary vulnerability to hypoxia when renal blood flow is decreased (renal ischemia)
- Renal sinus
- The inner portion of the kidney containing the renal calyces and renal pelvis
- The minor calyces draining from each renal papilla merge to form major calyces.
- The major calyces merge to form the renal pelvis, which represents the proximal portion of the ureter.
- Renal hilum: The medial fissure on each kidney where the renal pelvis, vessels, and nerves enter and exit
Hormone synthesis
- Erythropoietin (EPO)
- Secreted by peritubular interstitial cells
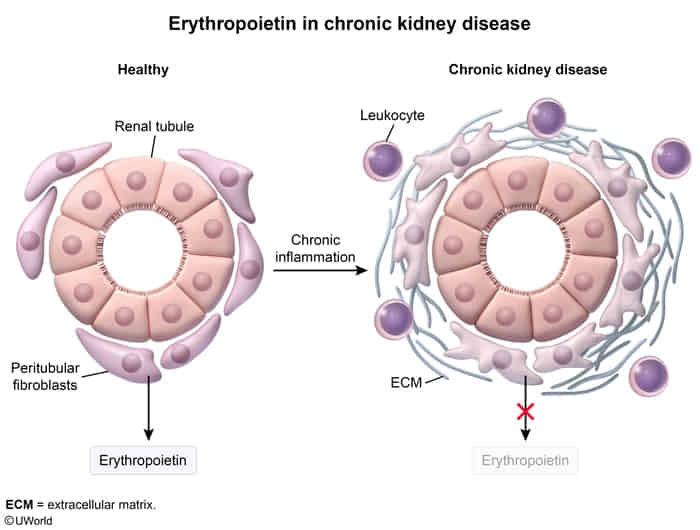
- Function: stimulates erythropoiesis in the bone marrow
- Regulation
- Positive feedback: anemia/blood loss, hypoxia
- Induced by the transcription factor HIF (hypoxia-inducible factor)
- Secreted by peritubular interstitial cells