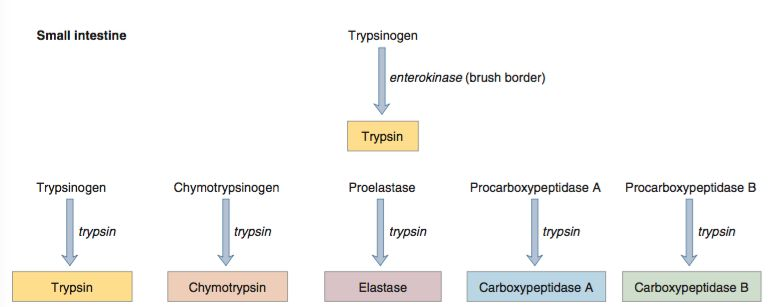
- Pancreatic enzymes (except amylase and lipase) are synthesized and secreted in an inactive form to protect the pancreas from autodigestion.
- These proenzymes (zymogens) are activated by trypsin in the duodenal lumen.
- Trypsinogen is converted into active trypsin by enteropeptidase (duodenal brush border enzyme).
- Works best in the slightly alkaline pH (~8.0)
- Activation cascade:
- Trypsin can activate other trypsinogen molecules.
- Even small amounts of trypsin can trigger this cascade.
- Protective mechanisms to limit premature trypsinogen activation:
- Serine peptidase inhibitor Kazal-type 1 (SPINK1):
- Secreted by pancreatic acinar cells.
- Functions as a trypsin inhibitor, impeding prematurely activated trypsinogen molecules within the pancreas.
- Trypsin self-inhibition:
- Trypsin can cleave active trypsin molecules at a second site, rendering them inactive.
- Hereditary pancreatitis:
- Rare disorder caused by mutations in the trypsinogen or SPINK1 gene.
- Common mutation results in abnormal trypsin that is not susceptible to inactivating cleavage by trypsin.
- Protective mechanisms are critical as small amounts of trypsinogen normally activate prematurely within pancreatic acini and ducts.
- Failure of these mechanisms leads to recurrent acute pancreatitis.
