Epidemiology
- Age of onset: individuals > 40 years
- Symptoms start to show when body iron levels reach > 20 g.
- Before menopause, women lose iron via menstruation and pregnancy, which slows down iron accumulation within the body. As a result, symptom onset occurs later in women (typically postmenopausal) than in men.
Etiology
Pathophysiology
HFE gene defect (homozygous) → defective binding of transferrin to its receptor → ↓ hepcidin synthesis by the liver → unregulated ferroportin activity in enterocytes → ↑ intestinal iron absorption → iron accumulation throughout the body → damage to the affected organs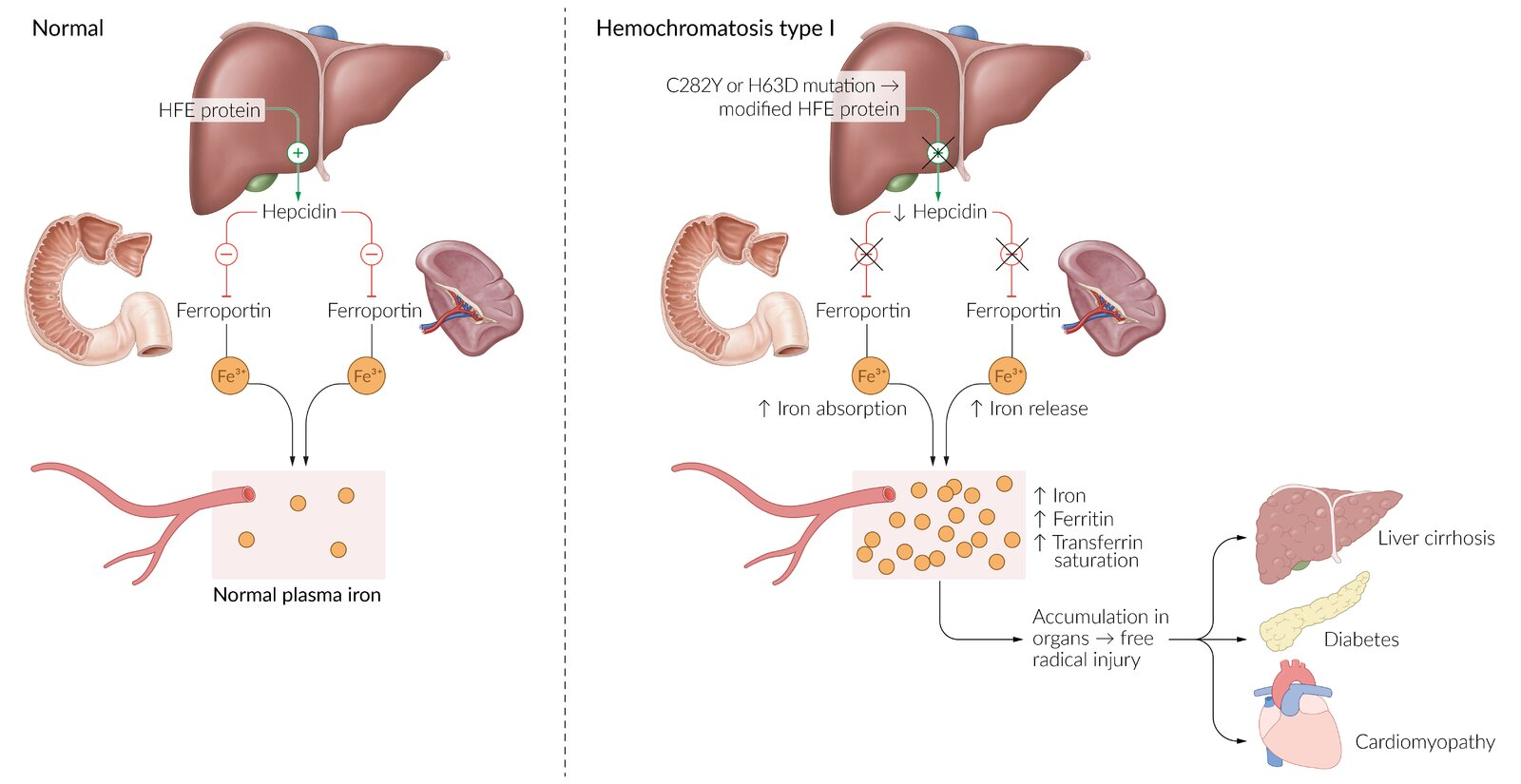
Tip
Don’t mess up with Wilson disease
- Wilson disease is accumulation of copper, and has neurologic symptoms.
Clinical features
Tip
Classic triad of cirrhosis, diabetes mellitus, skin pigmentation (“bronze diabetes”).
- Often asymptomatic for decades; men typically present earlier (age 40-60) than women due to iron loss from menstruation and pregnancy.
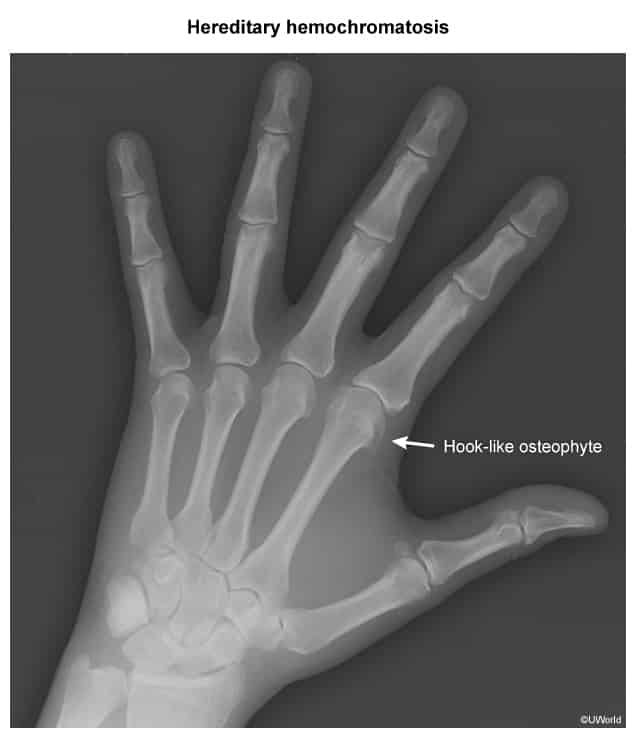
- Early symptoms are nonspecific: fatigue, arthralgia (especially in the 2nd and 3rd MCP joints, the “iron fist”), and decreased libido.
- Classic (but now rare) triad: “Bronze Diabetes”
- Cirrhosis: due to iron deposition in the liver.
- Diabetes Mellitus: due to iron deposition in pancreatic islet cells.
- Skin Hyperpigmentation: a bronze or slate-gray hue.
- Other manifestations: hypogonadism (pituitary iron deposition), cardiomyopathy (restrictive or dilated), and increased susceptibility to infections with iron-loving organisms (Vibrio vulnificus, Listeria, Yersinia).
| Feature | Hemochromatosis (Iron Overload) | Wilson Disease (Copper Overload) |
|---|---|---|
| Gene Defect | HFE gene (Autosomal Recessive) → ↑ Iron absorption. | ATP7B gene (Autosomal Recessive) → ↓ Copper excretion. |
| ”Classic” Triad / Signs | 1. Cirrhosis (↑ HCC risk) 2. Diabetes Mellitus 3. Skin Pigmentation (“Bronze Diabetes”) | 1. Liver Disease (Hepatitis, Cirrhosis) 2. Neurologic Dysfunction (Parkinsonism, tremor) 3. Kayser-Fleischer Rings (Ocular) |
| Key Labs | ↑ Ferritin ↑ Iron ↑ Transferrin Saturation | ↓ Ceruloplasmin ↑ Urinary Copper |
| Treatment | Phlebotomy Iron Chelators (Deferasirox) | Copper Chelators (Penicillamine, Trientine) Oral Zinc |
Diagnostics
- Liver biopsy
- Hemosiderin (normally golden yellow on microscopy) appears blue with the Prussian blue stain.
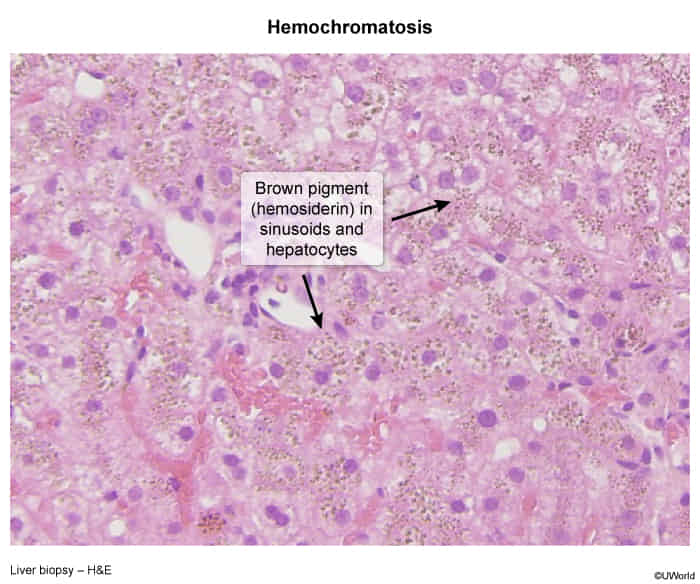
- Pattern of hereditary hemochromatosis: pronounced parenchymal siderosis (accumulation of hemosiderin within the tissue) in hepatocytes and bile duct epithelium
- Pattern of secondary iron overload: Kupffer cells (specialized macrophages) containing hemosiderin
- Hemosiderin (normally golden yellow on microscopy) appears blue with the Prussian blue stain.
Treatment
Iron chelation therapy
- First-line treatment for secondary iron overload due to iron-loading anemia
- Chelating agents: deferoxamine