Epidemiology
Etiology
- Idiopathic inflammatory autoimmune disorder of unknown etiology
- Risk factors include:
- Genetic disposition: associated with HLA-DR4 and HLA-DR1
- Environmental factors (e.g., smoking)
- Hormonal factors (premenopausal women are at the highest risk, suggesting a predisposing role of female sex hormones)
Pathophysiology
- Certain interstitial tissue proteins (e.g. intracellular filament protein vimentin, filaggrin, type II collagen) undergo a posttranslational modification that involves the conversion of arginine to citrulline (citrullination).
- Citrullinated proteins are recognized as foreign by the antigen-presenting cells that present them to CD4+ T cells.
- Activation of CD4+ T cells leads to the following sequences of events:
- IL-4 production → B-cell proliferation and differentiation → production of anticitrullinated peptide antibodies → type II hypersensitivity reaction and type III hypersensitivity reaction
- Migration of CD4+ T cells to synovial joints → secretion of cytokines (IFN-γ, IL-17) → recruitment of macrophages → secretion of cytokines (TNF-α, IL-1, IL-6) → inflammation and proliferation
- Bouts of inflammation, angiogenesis, and proliferation → proliferative granulation tissue with mononuclear inflammatory cells → pannus and synovial hypertrophy → invasion, progressive destruction, and deterioration of cartilage and bone
- Pannus: latin, means cloth (the abnormal tissue growth in rheumatoid arthritis resembles a sheet or cloth-like layer spreading over the joint surface.)
- A granulation tissue growth in the inflamed synovium that causes it to become thickened and edematous in patients with chronic rheumatoid arthritis. It can cause fibrous ankylosis and/or joint deformities and the destruction of other intraarticular structures, such as cartilage.
- Pannus: latin, means cloth (the abnormal tissue growth in rheumatoid arthritis resembles a sheet or cloth-like layer spreading over the joint surface.)
- Antibodies against Fc portion of IgG (rheumatoid factor, RF) are produced to aid in removing autoantibodies and immune complexes.
- RF excess triggers formation of new immune complexes and type III HSR
- Individuals with positive RF are more likely to develop extraarticular manifestations.
Clinical features
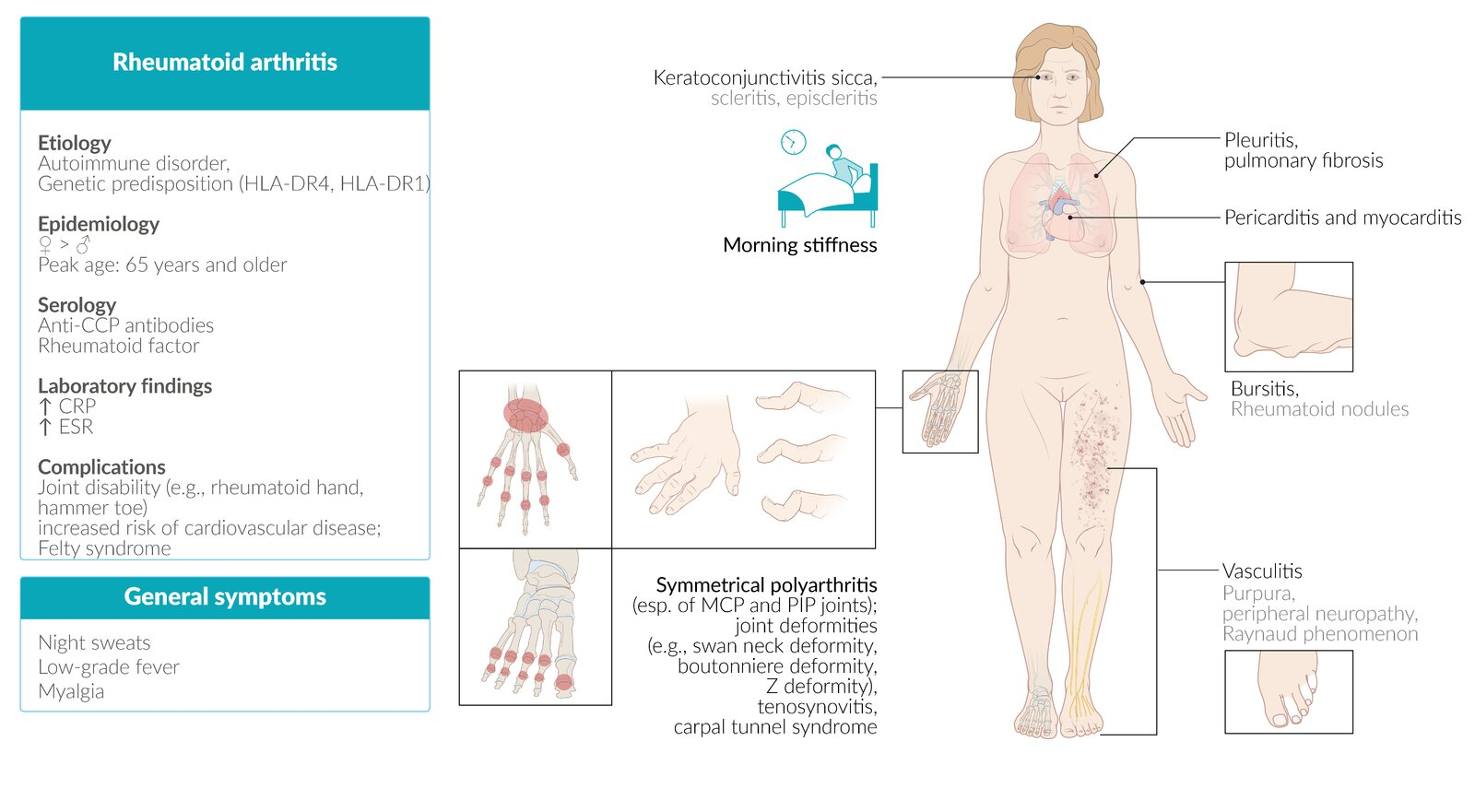
Extraarticular manifestations
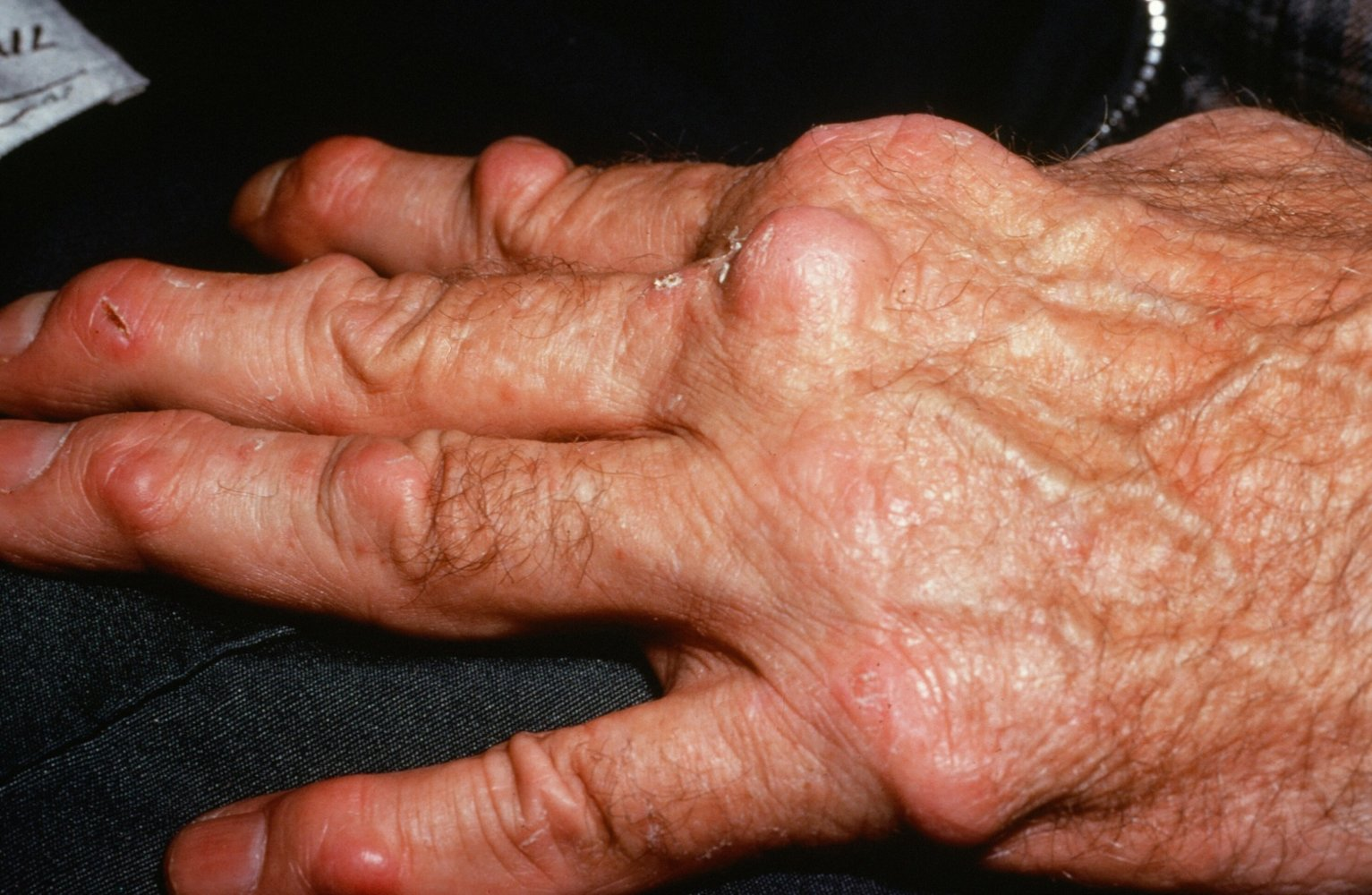
- Rheumatoid nodules
- Skin
- Nontender, firm, subcutaneous swellings (2 mm–5 cm)
- Commonly occur in areas exposed to higher pressure, e.g., extensor side of the forearm, bony prominences
- Lungs
- Typically bilateral and peripheral
- Rheumatoid pulmonary nodules may be accompanied by fibrosis and pneumoconiosis (Caplan syndrome).
- Skin
Subtypes and variants
Atlantoaxial subluxation (Vertebral subluxation)
- Definition: a potentially life-threatening complication caused by the inflammatory destruction of the ligaments affecting the atlantoaxial joint and the intervertebral joints
- Clinical features
- Pain and stiffness of the neck (typically early-morning neck pain at rest)
- Head tilt
- Neurological deficits
- Cervical radiculopathy with peripheral paresthesias of the upper limb
- In some cases, symptoms of high spinal cord compression
- Slowly progressive spastic quadriparesis
- Hyperreflexia or positive Babinski reflex
- Respiratory insufficiency
- Diagnostics
- Extension and flexion x-rays of the cervical spine
- MRI
Warning
Endotracheal intubation can acutely worsen the subluxation and cause compression of the spinal cord and/or vertebral arteries.
Diagnostics
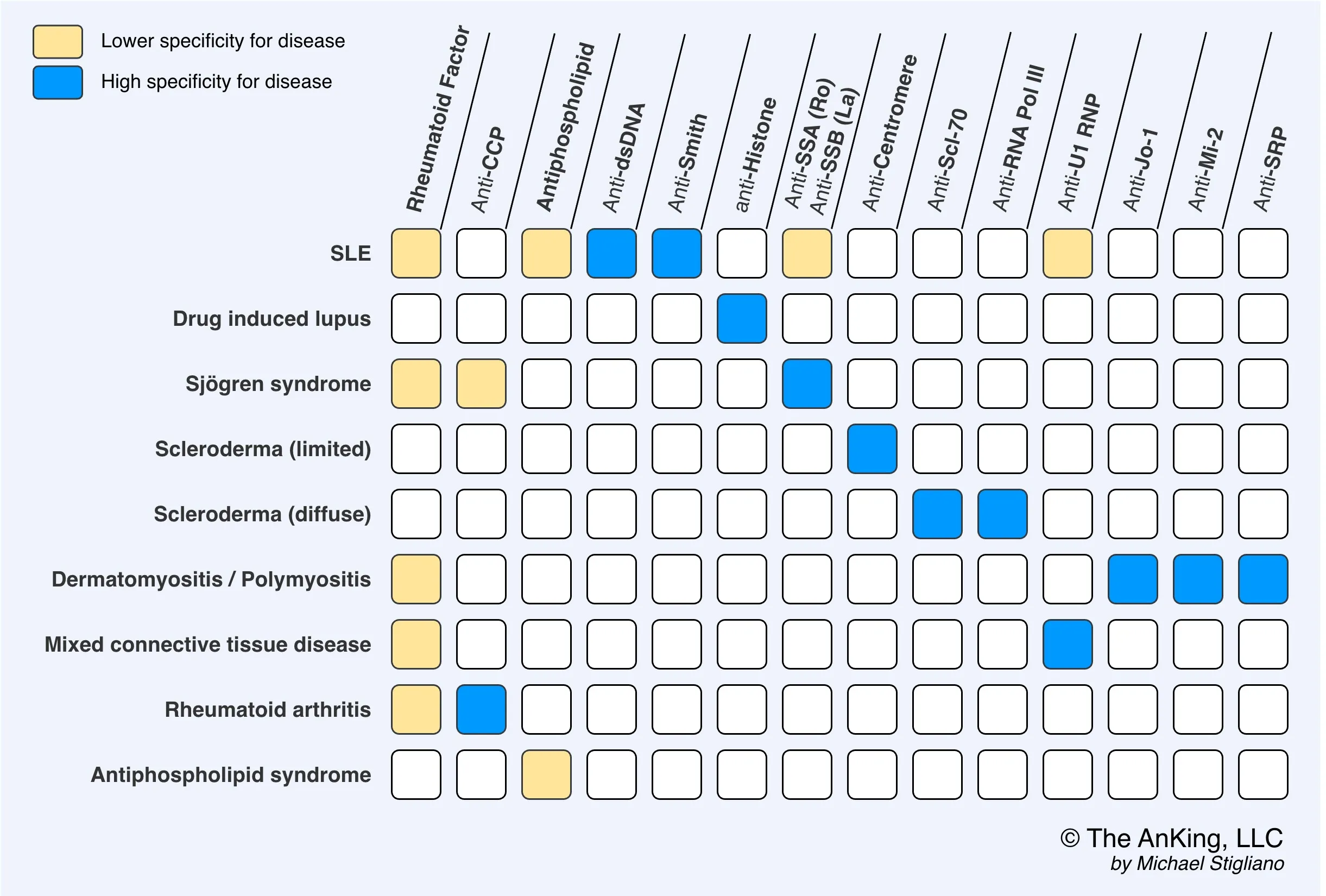
- Anticitrullinated peptide antibodies (ACPA), e.g., anticyclic citrullinated peptide (anti-CCP)
- Tissue inflammation causes arginine residues in proteins such as vimentin to be enzymatically converted into citrulline through a process called citrullination. This alters the shape of the proteins, which can then serve as neoantigens that generate an immune response.
- Rheumatoid factor (RF): IgM autoantibodies against the Fc region of IgG antibodies
Differential diagnostics
- Parvovirus B19 will presents with similar symptoms, except for rash, normal ESR, and acute onset
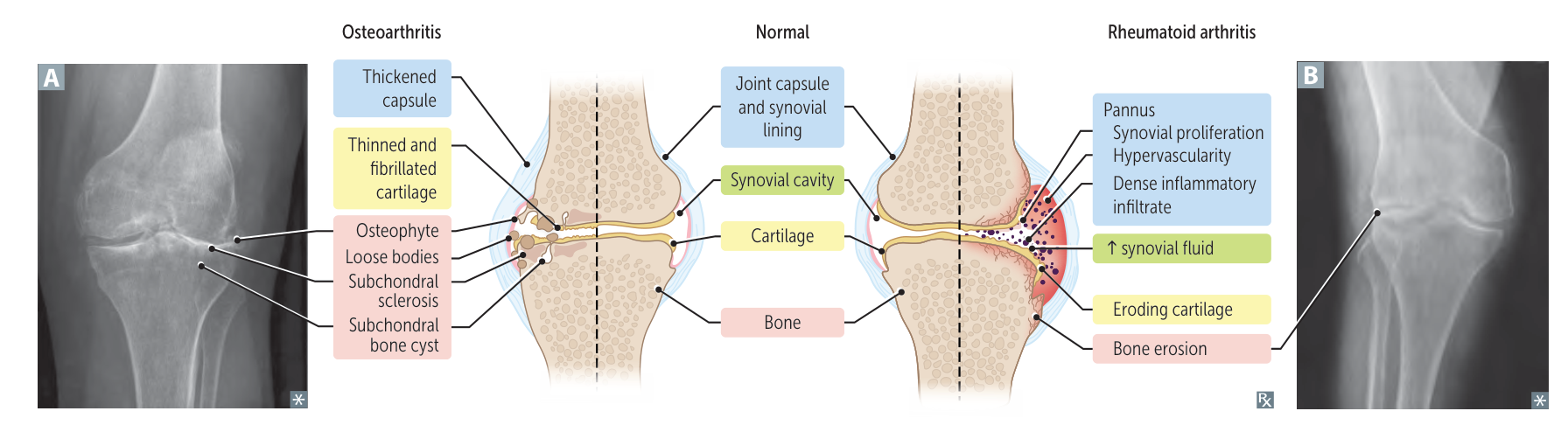
- See Osteoarthritis > Differential diagnosis
| Characteristic | Osteoarthritis (OA) | Rheumatoid Arthritis (RA) |
|---|---|---|
| Age of onset | >50 years | 30-50 years |
| Cause | ”Wear and tear” or trauma causing cartilage deterioration | Autoimmune inflammatory reaction against synovium |
| Primary joints affected | Weight-bearing joints (hips, knees), DIP, CMC of thumb | PIP, MCP, ankle, elbow, wrist; spares DIP Atlantoaxial subluxation |
| Joint characteristics | Hard and bony | Soft, warm, and tender |
| Pain pattern | Worse during or after activity | Worse in the morning or with inactivity |
| Stiffness | <30 minutes in morning, worse with activity | >30 minutes in morning, worse with inactivity |
| Joint symmetry | Often asymmetric, reflecting use patterns | Typically symmetric, diffuse involvement |
| Lab findings | Normal rheumatoid factor, normal anti-CCP antibody, normal ESR and CRP | Positive rheumatoid factor, positive anti-CCP antibody, elevated ESR and CRP |
| Associated signs | Heberden’s nodes (DIP), Bouchard’s nodes (PIP) | Ulnar deviation, boutonniere deformity, swan-neck deformity |
| Systemic involvement | None | Potential pulmonary and cardiac disease |
| Gender predilection | None | 2x more common in females |
| X-ray findings | Osteophytes, subchondral sclerosis, asymmetric joint space narrowing | Symmetric joint space loss, osteopenia, “apple coring” bone erosion |
| Exam findings | Effusion, tenderness | Effusion, tenderness, redness, warmth, synovitis |
Treatment
Acute anti-inflammatory treatment
- Glucocorticoids
- Systemic prednisone
- Longer-term therapy: Only use in patients with highly active RA
- Systemic prednisone
- NSAIDs and selective COX-2 inhibitors: relieve symptoms, but do not improve the prognosis
Long-term pharmacological treatment
Disease-modifying antirheumatic drugs (DMARDs)
- Methotrexate (MTX): first-line treatment in patients with moderate to high disease activity
- To minimize adverse effects, administer folic acid.
Biologic DMARDs
- Indication: persistent moderate or severe disease activity after 3 months of conventional DMARD therapy
- Agents
- TNF-α inhibitors: e.g., adalimumab, infliximab, etanercept
Complications
- Amyloidosis > AA amyloidosis (secondary amyloidosis)
- Septic arthritis